
Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.

Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.

Scandium(III) chloride is the inorganic compound with the formula ScCl3. It is a white, high-melting ionic compound, which is deliquescent and highly water-soluble. This salt is mainly of interest in the research laboratory. Both the anhydrous form and hexahydrate (ScCl3•6H2O) are commercially available.

Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.

Uranium(III) chloride, UCl3, is a chemical compound that contains the earth metal uranium and chlorine. UCl3 is used mostly to reprocess spent nuclear fuel. Uranium(III) chloride is synthesized in various ways from uranium(IV) chloride; however, UCl3 is less stable than UCl4.

Vanadium trichloride is the inorganic compound with the formula VCl3. This purple salt is a common precursor to other vanadium(III) complexes.

Organoactinide chemistry is the science exploring the properties, structure and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.
Niobocene dichloride is the organometallic compound with the formula (C5H5)2NbCl2, abbreviated Cp2NbCl2. This paramagnetic brown solid is a starting reagent for the synthesis of other organoniobium compounds. The compound adopts a pseudotetrahedral structure with two cyclopentadienyl and two chloride substituents attached to the metal. A variety of similar compounds are known, including Cp2TiCl2.

Niobium pentoxide is the inorganic compound with the formula Nb2O5. It is a colorless insoluble solid that is fairly unreactive. It is the main precursor to all materials made of niobium, the dominant application being alloys, but other specialized applications include capacitors, lithium niobate, and optical glasses.

Niobium(V) bromide is the inorganic compound with the formula Nb2Br10. Its name comes from the compound's empirical formula, NbBr5. It is a diamagnetic, orange solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two NbBr5 units are joined by a pair of bromide bridges. There is no bond between the Nb centres. Niobium(V) chloride, niobium(V) iodide, tantalum(V) chloride, tantalum(V) bromide, and tantalum(V) iodide all share this structural motif.
Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon to vanadium (V) chemical bond. Organovanadium compounds find only minor use as reagents in organic synthesis but are significant for polymer chemistry as catalysts.

Aluminium borohydride, also known as aluminium tetrahydroborate, (in American English, aluminum borohydride and aluminum tetrahydroborate, respectively) is the chemical compound with the formula Al(BH4)3. It is a volatile pyrophoric liquid which is used as rocket fuel, and as a reducing agent in laboratories. Unlike most other metal–borohydrides, which are ionic structures, aluminium borohydride is a covalent compound.

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides.
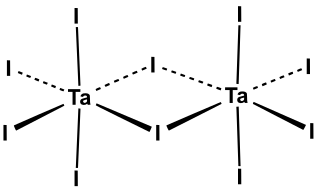
Tantalum(V) iodide is the inorganic compound with the formula Ta2I10. Its name comes from the compound's empirical formula, TaI5. It is a diamagnetic, black solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two TaI5 units are joined by a pair of iodide bridges. There is no bond between the Ta centres. Niobium(V) chloride, niobium(V) bromide, niobium(V) iodide, tantalum(V) chloride, and tantalum(V) bromide all share this structural motif.
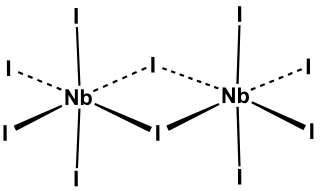
Niobium(V) iodide is the inorganic compound with the formula Nb2I10. Its name comes from the compound's empirical formula, NbI5. It is a diamagnetic, yellow solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two NbI5 units are joined by a pair of iodide bridges. There is no bond between the Nb centres. Niobium(V) chloride, niobium(V) bromide, tantalum(V) chloride, tantalum(V) bromide, and tantalum(V) iodide, all share this structural motif.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).
Organoniobium chemistry is the chemistry of compounds containing niobium-carbon (Nb-C) bonds. Compared to the other group 5 transition metal organometallics, the chemistry of organoniobium compounds most closely resembles that of organotantalum compounds. Organoniobium compounds of oxidation states +5, +4, +3, +2, +1, 0, -1, and -3 have been prepared, with the +5 oxidation state being the most common.
Niobium(III) chloride also known as niobium trichloride is a compound of niobium and chlorine. The binary phase NbCl3 is not well characterized but many adducts are known.
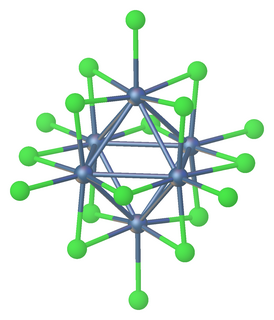
Tantalum(III) chloride or tantalum trichloride is non-stoichiometric with a range of composition from TaCl2.9 to TaCl3.1 Anionic and neutral clusters containing Ta(III) chloride include [Ta6Cl18]4− and [Ta6Cl14](H2O)4.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.

















