Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.
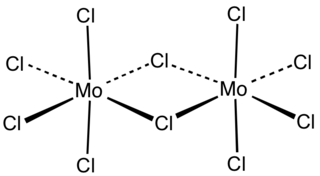
Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Tungsten hexachloride is an inorganic chemical compound of tungsten and chlorine with the chemical formula WCl6. This dark violet blue compound exists as volatile crystals under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known.

Tungsten(V) chloride is an inorganic compound with the formula W2Cl10. This compound is analogous in many ways to the more familiar molybdenum pentachloride.

Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. The material exists as two polymorphs, both being dark-colored paramagnetic solids. These compounds are mainly of interest as precursors to other molybdenum complexes.
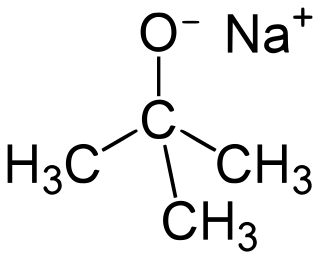
Sodium tert-butoxide (or sodium t-butoxide) is a chemical compound with the formula (CH3)3CONa (abbr. NaOtBu). It is a strong, non-nucleophilic base. It is flammable and moisture sensitive. It is sometimes written in the chemical literature as sodium t-butoxide. It is similar in reactivity to the more common potassium tert-butoxide.

Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.

trans-Bis(dinitrogen)bis[1,2-bis(diphenylphosphino)ethane]molybdenum(0) is a coordination complex with the formula Mo(N2)2(dppe)2. It is a relatively air stable yellow-orange solid. It is notable as being the first discovered dinitrogen containing complex of molybdenum.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.

Tungsten(II) chloride is the inorganic compound with the formula W6Cl12. It is a polymeric cluster compound. The material dissolves in concentrated hydrochloric acid, forming (H3O)2[W6Cl14](H2O)x. Heating this salt gives yellow-brown W6Cl12. The structural chemistry resembles that observed for molybdenum(II) chloride.

Tungsten(III) chloride is the inorganic compound with the formula W6Cl18. It is a cluster compound. It is a brown solid, obtainable by chlorination of tungsten(II) chloride. Featuring twelve doubly bridging chloride ligands, the cluster adopts a structure related to the corresponding chlorides of niobium and tantalum. In contrast, W6Cl12 features eight triply bridging chlorides.

Rhenium(IV) chloride is the inorganic compound with the formula ReCl4. This black solid is of interest as a binary phase but otherwise is of little practical value. A second polymorph of ReCl4 is also known.

Tantalum(III) chloride or tantalum trichloride is non-stoichiometric chemical compound with a range of composition from TaCl2.9 to TaCl3.1 Anionic and neutral clusters containing Ta(III) chloride include [Ta6Cl18]4− and [Ta6Cl14](H2O)4.
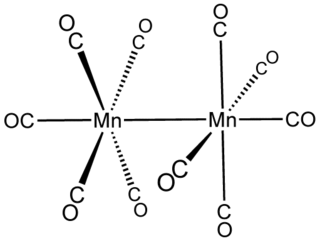
In inorganic chemistry, metal–metal bonds describe attractive interactions between metal centers. The simplest examples are found in bimetallic complexes. Metal–metal bonds can be "supported", i.e. be accompanied by one or more bridging ligands, or "unsupported". They can also vary according to bond order. The topic of metal–metal bonding is usually discussed within the framework of coordination chemistry, but the topic is related to extended metallic bonding, which describes interactions between metals in extended solids such as bulk metals and metal subhalides.

Hexa(tert-butoxy)ditungsten(III) is a coordination complex of tungsten(III). It is one of the homoleptic alkoxides of tungsten. A red, air-sensitive solid, the complex has attracted academic attention as the precursor to many organotungsten derivatives. It an example of a charge-neutral complex featuring a W≡W bond, arising from the coupling of a pair of d3 metal centers. It has attracted particular attention for its reactions with alkynes, leading to alkyne metathesis.

Lithium tert-butoxide is the metalorganic compound with the formula LiOC(CH3)3. A white solid, it is used as a strong base in organic synthesis. The compound is often depicted as a salt, and it often behaves as such, but it is not ionized in solution. Both octameric and hexameric forms have been characterized by X-ray crystallography
In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.