In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

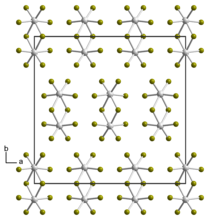
Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques.

Palladium(II) acetate is a chemical compound of palladium described by the formula [Pd(O2CCH3)2]n, abbreviated [Pd(OAc)2]n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions.

Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Tungsten hexachloride is an inorganic chemical compound of tungsten and chlorine with the chemical formula WCl6. This dark violet-blue compound exists as volatile crystals under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known.

Vanadium(III) bromide, also known as vanadium tribromide, describes the inorganic compounds with the formula VBr3 and its hydrates. The anhydrous material is a green-black solid. In terms of its structure, the compound is polymeric with octahedral vanadium(III) surrounded by six bromide ligands.

Methyllithium is the simplest organolithium reagent, with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive towards water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.

Tantalum(V) bromide is the inorganic compound with the formula Ta2Br10. Its name comes from the compound's empirical formula, TaBr5. It is a diamagnetic, orange solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two TaBr5 units are joined by a pair of bromide bridges. There is no bond between the Ta centres. Niobium(V) chloride, niobium(V) bromide, niobium(V) iodide, tantalum(V) chloride, and tantalum(V) iodide all share this structural motif.

Niobium(IV) chloride, also known as niobium tetrachloride, is the chemical compound of formula NbCl4. This compound exists as dark violet crystals, is highly sensitive to air and moisture, and disproportiates into niobium(III) chloride and niobium(V) chloride when heated.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride.

Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.

Zirconium(III) bromide is an inorganic compound with the formula ZrBr3.

Zirconium(III) iodide is an inorganic compound with the formula ZrI3.
Protactinium(V) bromide is an inorganic compound. It is a halide of protactinium, consisting of protactinium and bromine. It is radioactive and has a chemical formula of PaBr5, which is a red crystal of the monoclinic crystal system.

Protactinium(IV) bromide is an inorganic compound. It is an actinide halide, composed of protactinium and bromine. It is radioactive, and has the chemical formula of PaBr4. It may be due to the brown color of bromine that causes the appearance of protactinium(IV) bromide to be brown crystals. Its crystal structure is tetragonal. Protactinium(IV) bromide is sublimed in a vacuum at 400 °C. The protactinium(IV) halide closest in structure to protactinium(IV) bromide is protactinium(IV) chloride.
Protactinium compounds are compounds containing the element protactinium. These compounds usually have protactinium in the +5 oxidation state, although these compounds can also exist in the +2, +3 and +4 oxidation states.
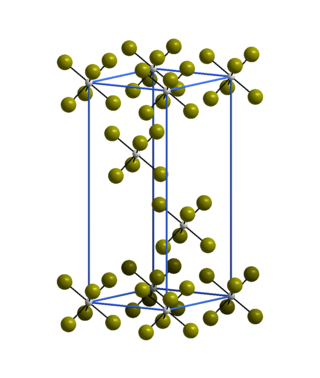
Tungsten hexabromide, also known as tungsten(VI) bromide, is a chemical compound of tungsten and bromine with the formula WBr6. It is an air-sensitive dark grey powder that decomposes above 200 °C to tungsten(V) bromide and bromine.
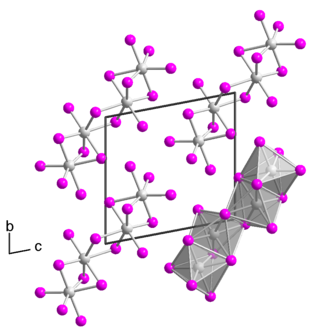
Tantalum(IV) iodide is an inorganic compound with the chemical formula TaI4. It dissolves in water to give a green solution, but the color fades when left in the air and produces a white precipitate.