In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.
Zirconium(IV) bromide is the inorganic compound with the formula ZrBr4. This colourless solid is the principal precursor to other Zr–Br compounds.

Gallium(III) bromide (GaBr3) is a chemical compound, and one of four gallium trihalides.
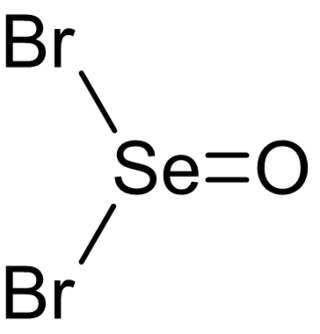
Selenium oxybromide (SeOBr2) is a selenium oxohalide chemical compound.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.

Silicon tetrabromide, also known as tetrabromosilane, is the inorganic compound with the formula SiBr4. This colorless liquid has a suffocating odor due to its tendency to hydrolyze with release of hydrogen bromide. The general properties of silicon tetrabromide closely resemble those of the more commonly used silicon tetrachloride.
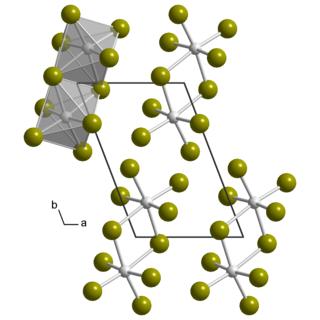
Uranium pentabromide is an inorganic chemical compound with the formula U2Br10.

Germanium(IV) iodide is an inorganic compound with the chemical formula GeI4.

NASICON is an acronym for sodium (Na) super ionic conductor, which usually refers to a family of solids with the chemical formula Na1+xZr2SixP3−xO12, 0 < x < 3. In a broader sense, it is also used for similar compounds where Na, Zr and/or Si are replaced by isovalent elements. NASICON compounds have high ionic conductivities, on the order of 10−3 S/cm, which rival those of liquid electrolytes. They are caused by hopping of Na ions among interstitial sites of the NASICON crystal lattice.
Germanium(II) hydrides, also called germylene hydrides, are a class of Group 14 compounds consisting of low-valent germanium and a terminal hydride. They are also typically stabilized by an electron donor-acceptor interaction between the germanium atom and a large, bulky ligand.
Molybdenum(IV) bromide, also known as molybdenum tetrabromide, is the inorganic compound with the formula MoBr4. It is a black solid. MoBr4 has been prepared by treatment of molybdenum(V) chloride with hydrogen bromide:

Selenium tetrabromide is an inorganic compound with a chemical formula SeBr4.

Lithium aluminium germanium phosphate, typically known with the acronyms LAGP or LAGPO, is an inorganic ceramic solid material whose general formula is Li
1+xAl
xGe
2-x(PO
4)
3. LAGP belongs to the NASICON family of solid conductors and has been applied as a solid electrolyte in all-solid-state lithium-ion batteries. Typical values of ionic conductivity in LAGP at room temperature are in the range of 10–5 - 10–4 S/cm, even if the actual value of conductivity is strongly affected by stoichiometry, microstructure, and synthesis conditions. Compared to lithium aluminium titanium phosphate (LATP), which is another phosphate-based lithium solid conductor, the absence of titanium in LAGP improves its stability towards lithium metal. In addition, phosphate-based solid electrolytes have superior stability against moisture and oxygen compared to sulfide-based electrolytes like Li
10GeP
2S
12 (LGPS) and can be handled safely in air, thus simplifying the manufacture process. Since the best performances are encountered when the stoichiometric value of x is 0.5, the acronym LAGP usually indicates the particular composition of Li
1.5Al
0.5Ge
1.5(PO
4)
3, which is also the typically used material in battery applications.
Osmium tetrabromide is the inorganic compound with the formula OsBr4. A black solid, this compound can be produced by heating osmium tetrachloride and bromine under pressure.
Sulfidogermanates or thiogermanates are chemical compounds containing anions with sulfur atoms bound to germanium. They are in the class of chalcogenidotetrelates. Related compounds include thiosilicates, thiostannates, selenidogermanates, telluridogermanates and selenidostannates.
Ytterbium compounds are chemical compounds that contain the element ytterbium (Yb). The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium, and thulium, the trihalides of ytterbium can be reduced to the dihalides by hydrogen, zinc dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide (CaO).

Germanium dibromide is a bromide of germanium with the chemical formula GeBr2.
Thorium(IV) bromide is an inorganic compound, with the chemical formula of ThBr4.










