Germanium is a chemical element; it has symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature.
A metalloid is a chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. The word metalloid comes from the Latin metallum ("metal") and the Greek oeides. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature.

In the context of the periodic table a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter than elements that form metals and are often poor conductors of heat and electricity. Chemically, nonmetals have relatively high electronegativity or usually attract electrons in a chemical bond with another element, and their oxides tend to be acidic.

Silver sulfide is an inorganic compound with the formula Ag
2S. A dense black solid, it is the only sulfide of silver. It is useful as a photosensitizer in photography. It constitutes the tarnish that forms over time on silverware and other silver objects. Silver sulfide is insoluble in most solvents, but is degraded by strong acids. Silver sulfide is a network solid made up of silver and sulfur where the bonds have low ionic character.

Tungsten(VI) fluoride, also known as tungsten hexafluoride, is an inorganic compound with the formula WF6. It is a toxic, corrosive, colorless gas, with a density of about 13 kg/m3 (22 lb/cu yd). It is the only known gaseous transition metal compound and the densest known gas under standard ambient temperature and pressure. WF6 is commonly used by the semiconductor industry to form tungsten films, through the process of chemical vapor deposition. This layer is used in a low-resistivity metallic "interconnect". It is one of seventeen known binary hexafluorides.

Mercury sulfide, or mercury(II) sulfide is a chemical compound composed of the chemical elements mercury and sulfur. It is represented by the chemical formula HgS. It is virtually insoluble in water.

Germanium tetrachloride is a colourless, fuming liquid with a peculiar, acidic odour. It is used as an intermediate in the production of purified germanium metal. In recent years, GeCl4 usage has increased substantially due to its use as a reagent for fiber optic production.

Tin selenide, also known as stannous selenide, is an inorganic compound with the formula SnSe. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor structurally analogous to black phosphorus. It has received considerable interest for possible applications including low-cost photovoltaics, and memory-switching devices. Because of its low thermal conductivity as well as reasonable electrical conductivity, tin selenide is one of the most efficient thermoelectric materials.
Germanium dioxide, also called germanium(IV) oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula GeO2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germanium in contact with atmospheric oxygen.
Germanium iodides are inorganic compound with the formula GeIx. Two such compounds exist: germanium(II) iodide, GeI2, and germanium(IV) iodide GeI4.

Germanium dichloride is a chemical compound of germanium and chlorine with the formula GeCl2. It is a yellow solid. Germanium dichloride is an example of a compound featuring germanium in the +2 oxidation state.

Antimony telluride is an inorganic compound with the chemical formula Sb2Te3. As is true of other pnictogen chalcogenide layered materials, it is a grey crystalline solid with layered structure. Layers consist of two atomic sheets of antimony and three atomic sheets of tellurium and are held together by weak van der Waals forces. Sb2Te3 is a narrow-gap semiconductor with a band gap 0.21 eV; it is also a topological insulator, and thus exhibits thickness-dependent physical properties.

Germanium tetrafluoride (GeF4) is a chemical compound of germanium and fluorine. It is a colorless gas.

Germanium difluoride (GeF2) is a chemical compound of germanium and fluorine. It is a white solid with a melting point of 110 °C, and can be produced by reacting germanium tetrafluoride with germanium powder at 150–300 °C.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.

Germanium(II) iodide is an iodide of germanium, with the chemical formula of GeI2.

Silicon monosulfide is a chemical compound of silicon and sulfur. The chemical formula is SiS. Molecular SiS has been detected at high temperature in the gas phase. The gas phase molecule has an Si-S bondlength of 192.93 pm, this compares to the normal single bond length of 216 pm, and is shorter than the Si=S bond length of around 201 pm reported in an organosilanethione. Historically a pale yellow-red amorphous solid compound has been reported. The behavior of silicon can be contrasted with germanium which forms a stable solid monosulfide.
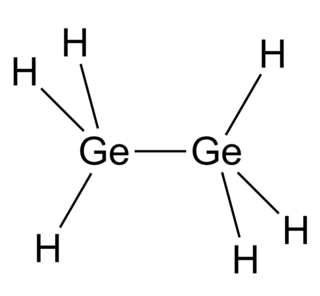
Digermane is an inorganic compound with the chemical formula Ge2H6. One of the few hydrides of germanium, it is a colourless liquid. Its molecular geometry is similar to ethane.

The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metals, other metals, p-block metals, basic metals, and chemically weak metals. The most common name, post-transition metals, is generally used in this article.
Hafnium compounds are compounds containing the element hafnium (Hf). Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4. Halogens react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Some compounds of hafnium in lower oxidation states are known.