
Pentlandite is an iron–nickel sulfide with the chemical formula (Fe,Ni)9S8. Pentlandite has a narrow variation range in nickel to iron ratios (Ni:Fe), but it is usually described as 1:1. In some cases, this ratio is skewed by the presence of pyrrhotite inclusions. It also contains minor cobalt, usually at low levels as a fraction of weight.

Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as Hydrous ferric oxide.
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when the populations consist of different atoms or ions (such as Fe2+ and Fe3+).

Sekaninaite ((Fe+2,Mg)2Al4Si5O18) is a silicate mineral, the iron-rich analogue of cordierite.

Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the exception of extremely rare native iron deposits, it is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism.

Maghemite (Fe2O3, γ-Fe2O3) is a member of the family of iron oxides. It has the same spinel ferrite structure as magnetite and is also ferrimagnetic. It is sometimes spelled as "maghaemite".

Pyrrhotite is an iron sulfide mineral with the formula Fe(1-x)S. It is a nonstoichiometric variant of FeS, the mineral known as troilite. Pyrrhotite is also called magnetic pyrite, because the color is similar to pyrite and it is weakly magnetic. The magnetism decreases as the iron content decreases, and troilite is non-magnetic. Pyrrhotite is generally tabular and brassy/bronze in color with a metallic luster. The mineral occurs with mafic igneous rocks like norites, and may form from pyrite during metamorphic processes. Pyrrhottie is associated and mined with other sulfide minerals like pentlandite, pyrite, chalcopyrite, and magnetite, and has been found globally.

Clay minerals are hydrous aluminium phyllosilicates (e.g. kaolin, Al2Si2O5(OH)4), sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations found on or near some planetary surfaces.

Iron(II,III) oxide, or black iron oxide, is the chemical compound with formula Fe3O4. It occurs in nature as the mineral magnetite. It is one of a number of iron oxides, the others being iron(II) oxide (FeO), which is rare, and iron(III) oxide (Fe2O3) which also occurs naturally as the mineral hematite. It contains both Fe2+ and Fe3+ ions and is sometimes formulated as FeO ∙ Fe2O3. This iron oxide is encountered in the laboratory as a black powder. It exhibits permanent magnetism and is ferrimagnetic, but is sometimes incorrectly described as ferromagnetic. Its most extensive use is as a black pigment (see: Mars Black). For this purpose, it is synthesized rather than being extracted from the naturally occurring mineral as the particle size and shape can be varied by the method of production.
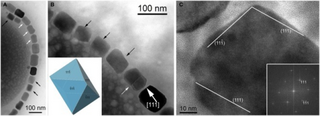
Magnetosomes are membranous structures present in magnetotactic bacteria (MTB). They contain iron-rich magnetic particles that are enclosed within a lipid bilayer membrane. Each magnetosome can often contain 15 to 20 magnetite crystals that form a chain which acts like a compass needle to orient magnetotactic bacteria in geomagnetic fields, thereby simplifying their search for their preferred microaerophilic environments. Recent research has shown that magnetosomes are invaginations of the inner membrane and not freestanding vesicles. Magnetite-bearing magnetosomes have also been found in eukaryotic magnetotactic algae, with each cell containing several thousand crystals.

A ferrite is a ceramic material made by mixing and firing large proportions of iron(III) oxide blended with small proportions of one or more additional metallic elements, such as strontium, barium, manganese, nickel, and zinc. They are ferrimagnetic, meaning they can be magnetized or attracted to a magnet. Unlike other ferromagnetic materials, most ferrites are not electrically conductive, making them useful in applications like magnetic cores for transformers to suppress eddy currents. Ferrites can be divided into two families based on their resistance to being demagnetized.
Magnetotaxis is a process implemented by a diverse group of Gram-negative bacteria that involves orienting and coordinating movement in response to Earth's magnetic field. This process is mainly carried out by microaerophilic and anaerobic bacteria found in aquatic environments such as salt marshes, seawater, and freshwater lakes. By sensing the magnetic field, the bacteria are able to orient themselves towards environments with more favorable oxygen concentrations. This orientation towards more favorable oxygen concentrations allows the bacteria to reach these environments faster as opposed to random movement through Brownian motion.

Iron(III) phosphate, also ferric phosphate, is the inorganic compound with the formula FePO4. Several related materials are known, including four polymorphs of FePO4 and two polymorphs of the dihydrate FePO4·(H2O)2. These materials find few technical applications as well as occurring in the mineral kingdom.
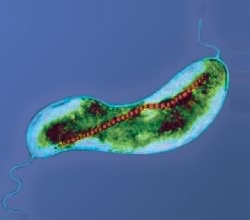
Magnetotactic bacteria are a polyphyletic group of bacteria that orient themselves along the magnetic field lines of Earth's magnetic field. Discovered in 1963 by Salvatore Bellini and rediscovered in 1975 by Richard Blakemore, this alignment is believed to aid these organisms in reaching regions of optimal oxygen concentration. To perform this task, these bacteria have organelles called magnetosomes that contain magnetic crystals. The biological phenomenon of microorganisms tending to move in response to the environment's magnetic characteristics is known as magnetotaxis. However, this term is misleading in that every other application of the term taxis involves a stimulus-response mechanism. In contrast to the magnetoreception of animals, the bacteria contain fixed magnets that force the bacteria into alignment—even dead cells are dragged into alignment, just like a compass needle.

Yttrium iron garnet (YIG) is a kind of synthetic garnet, with chemical composition Y3Fe2(FeO4)3, or Y3Fe5O12. It is a ferrimagnetic material with a Curie temperature of 560 K. YIG may also be known as yttrium ferrite garnet, or as iron yttrium oxide or yttrium iron oxide, the latter two names usually associated with powdered forms.

Greigite is an iron sulfide mineral with the chemical formula Fe2+Fe3+2S4. It is the sulfur equivalent of the iron oxide magnetite (Fe3O4). It was first described in 1964 for an occurrence in San Bernardino County, California, and named after the mineralogist and physical chemist Joseph W. Greig (1895–1977).

Mackinawite is an iron nickel sulfide mineral with the chemical formula (Fe,Ni)
1+xS. The mineral crystallizes in the tetragonal crystal system and has been described as a distorted, close packed, cubic array of S atoms with some of the gaps filled with Fe. Mackinawite occurs as opaque bronze to grey-white tabular crystals and anhedral masses. It has a Mohs hardness of 2.5 and a specific gravity of 4.17. It was first described in 1962 for an occurrence in the Mackinaw mine, Snohomish County, Washington for which it was named.

Magnetofossils are the fossil remains of magnetic particles produced by magnetotactic bacteria (magnetobacteria) and preserved in the geologic record. The oldest definitive magnetofossils formed of the mineral magnetite come from the Cretaceous chalk beds of southern England, while magnetofossil reports, not considered to be robust, extend on Earth to the 1.9-billion-year-old Gunflint Chert; they may include the four-billion-year-old Martian meteorite ALH84001.
Iron(III) sulfide, also known as ferric sulfide or sesquisulfide, is one of the several binary iron sulfides. It is a solid, black powder that degrades at ambient temperature.
Richard B. Frankel is an Emeritus Professor of Physics at the California State Polytechnic University, San Luis Obispo. He is noted for his research on magnetotaxis and biomineralization of magnetic iron minerals in general and magnetotactic bacteria in particular. His expertise in the latter was prominently discussed in Stephen Jay Gould's The Panda's Thumb. He is a graduate of the University of Missouri (1961) and took a PhD from Berkeley (1965). Much of his career was spent at the Francis Bitter National Magnet Laboratory, Massachusetts Institute of Technology before joining Cal Poly in 1988.



















