An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction

Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

Ammonium hydrosulfide is the chemical compound with the formula (NH4)HS.

Sodium sulfide is the chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts are colorless solids. They are water-soluble, giving strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, an extremely toxic, flammable and corrosive gas which smells like rotten eggs.

Phosphorus sulfides comprise a family of inorganic compounds containing only phosphorus and sulfur. These compounds have the formula P4Sx with x ≤ 10. Two are of commercial significance, phosphorus pentasulfide (P4S10), which is made on a kiloton scale for the production of other organosulfur compounds, and phosphorus sesquisulfide (P4S3), used in the production of "strike anywhere matches".

Sodium hydrosulfide is the chemical compound with the formula NaHS. This compound is the product of the half-neutralization of hydrogen sulfide (H2S) with sodium hydroxide. NaHS and sodium sulfide are used industrially, often for similar purposes. Solid NaHS is colorless. The solid has an odor H2S owing to hydrolysis by atmospheric moisture. In contrast with sodium sulfide (Na2S), which is insoluble in organic solvents, NaHS, being a 1:1 electrolyte, is more soluble.

Strontium bromide is a chemical compound with a formula SrBr2. At room temperature it is a white, odourless, crystalline powder. Strontium bromide imparts a bright red colour in a flame test, showing the presence of strontium ions. It is used in flares and also has some pharmaceutical uses.
Antimony pentasulfide is an inorganic compound of antimony and sulfur, also known as antimony red. It is a nonstoichiometric compound with a variable composition. Its exact structure is unknown. Commercial samples are usually contaminated with sulfur, which may be removed by washing with carbon disulfide in a Soxhlet extractor.

Arsenic pentasulfide is an inorganic compound contains arsenic and sulfur. The identity of this reddish solid remains uncertain.

Potassium hydrosulfide is the inorganic compound with the formula KHS. This colourless salt consists of the cation K+ and the bisulfide anion [SH]−. It is the product of the half-neutralization of hydrogen sulfide with potassium hydroxide. The compound is used in the synthesis of some organosulfur compounds. It is prepared by neutralizing aqueous KOH with H2S. Aqueous solutions of potassium sulfide consist of a mixture of potassium hydrosulfide and potassium hydroxide.
Acid strength is the tendency of an acid, symbolised by the chemical formula , to dissociate into a proton, , and an anion, . The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions.

Sodium polysulfide is a general term for salts with the formula Na2Sx, where x = 2 to 5. The species Sx2−, called polysulfide anions, include disulfide (S22−), trisulfide (S32−), tetrasulfide (S42−), and pentasulfide (S52−). In principle, but not in practice, the chain lengths could be longer. The salts are dark red solids that dissolve in water to give highly alkaline and corrosive solutions. In air, these salts oxidize, and they evolve hydrogen sulfide by hydrolysis.
Rubidium hydrogen sulfate is the rubidium salt of sulfuric acid. It has the formula RbHSO4.

Neptunium(IV) fluoride or neptunium tetrafluoride is a inorganic compound with the formula NpF4. It is a green salt and is isostructural with UF4.
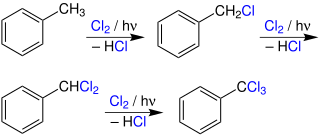
Photochlorination is a chlorination reaction that is initiated by light. Usually a C-H bond is converted to a C-Cl bond. Photochlorination is carried out on an industrial scale. The process is exothermic and proceeds as a chain reaction initiated by the homolytic cleavage of molecular chlorine into chlorine radicals by ultraviolet radiation. Many chlorinated solvents are produced in this way.
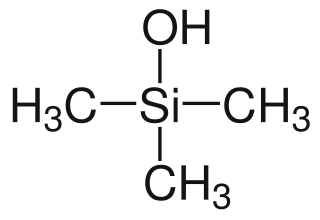
Organosilanols are a group of chemical silicon compounds. More specifically, they are carbosilanes derivatized with a hydroxy group on the silicon atom. Organosilanols are the silicon analogs to alcohols. Silanols are more acidic and more basic than their alcohol counterparts and therefore show a rich structural chemistry characterized by hydrogen bonding networks which are particularly well studied for silanetriols.

Rubidium azide is an inorganic compound with the formula RbN3. It is the rubidium salt of the azide ion (N–
3). Like most azides, it is explosive.

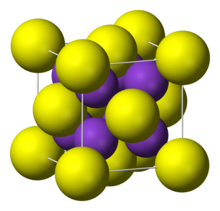
Potassium telluride is an inorganic compound with a chemical formula K2Te. It is formed from potassium and tellurium, making it a telluride. Potassium telluride is a white powder. Like rubidium telluride and caesium telluride, it can be used as an ultraviolet detector in space. Its crystal structure is similar to other tellurides, which have an anti-fluorite structure.

Caesium sulfide (also spelled cesium sulfide in American English) is an inorganic salt with a chemical formula Cs2S. It is a strong alkali in aqueous solution. In the air, caesium sulfide emits rotten egg smelling hydrogen sulfide.
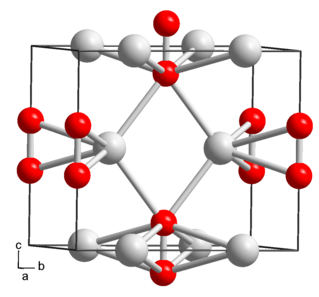
Rubidium peroxide is rubidium's peroxide with the chemical formula Rb2O2.