
Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.
Hydrobromic acid is an aqueous solution of hydrogen bromide. It is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C (255.7 °F) and contains 47.6% HBr by mass, which is 8.77 mol/L. Hydrobromic acid is one of the strongest mineral acids known.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C (255.7 °F). Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant materials, and cell stains. Although uncommon, chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption, see potassium bromide. The bromide ion has an ionic radius of 196 pm.
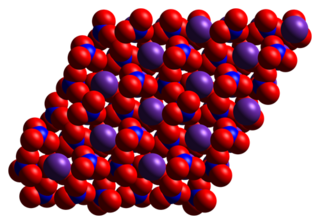
Rubidium nitrate is an inorganic compound with the formula RbNO3. This alkali metal nitrate salt is white and highly soluble in water.

Lithium bromide (LiBr) is a chemical compound of lithium and bromine. Its extreme hygroscopic character makes LiBr useful as a desiccant in certain air conditioning systems.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.
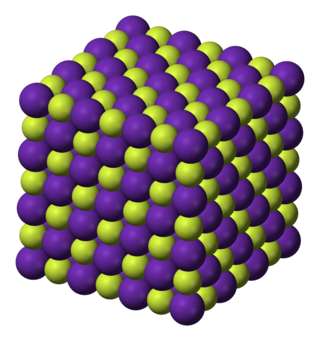
Rubidium fluoride (RbF) is the fluoride salt of rubidium. It is a cubic crystal with rock-salt structure.

Zinc bromide (ZnBr2) is an inorganic compound with the chemical formula ZnBr2. It is a colourless salt that shares many properties with zinc chloride (ZnCl2), namely a high solubility in water forming acidic solutions, and good solubility in organic solvents. It is hygroscopic and forms a dihydrate ZnBr2·2H2O.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.

Iron(II) bromide refers to inorganic compounds with the chemical formula FeBr2(H2O)x. The anhydrous compound (x = 0) is a yellow or brownish-colored paramagnetic solid. The tetrahydrate is also known, all being pale colored solids. They are common precursor to other iron compounds.

Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, synthetic perfumes and other organic compounds. Physically, allyl bromide is a colorless liquid with an irritating and persistent smell, however, commercial samples are yellow or brown. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use.

Strontium bromide is a chemical compound with a formula SrBr2. At room temperature it is a white, odourless, crystalline powder. Strontium bromide imparts a bright red colour in a flame test, showing the presence of strontium ions. It is used in flares and also has some pharmaceutical uses.

Tetraethylammonium bromide (TEAB) is a quaternary ammonium compound with the chemical formula C8H20N+Br−, often written as "Et4N+Br−" in the chemical literature. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis.

Barium bromide is the chemical compound with the formula BaBr2. It is ionic and hygroscopic in nature.

Beryllium bromide is the chemical compound with the formula BeBr2. It is very hygroscopic and dissolves well in water. The compound is a polymer with tetrahedral coordinated Be centres.

Rubidium iodide is a salt of rubidium and iodine, with the chemical formula RbI. It is a white solid with a melting point of 642 °C.

Bromous acid is the inorganic compound with the formula of HBrO2. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.
An yttrium compound is a chemical compound containing yttrium. Among these compounds, yttrium generally has a +3 valence. The solubility properties of yttrium compounds are similar to those of the lanthanides. For example oxalates and carbonates are hardly soluble in water, but soluble in excess oxalate or carbonate solutions as complexes are formed. Sulfates and double sulfates are generally soluble. They resemble the "yttrium group" of heavy lanthanide elements.














