 | |
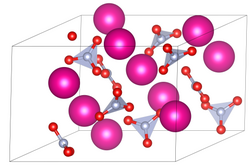 Unit cell of rubidium nitrate | |
| Names | |
|---|---|
| IUPAC name Rubidium nitrate | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.032.767 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| RbNO3 | |
| Molar mass | 147.473 g/mol |
| Appearance | White hygroscopic solid |
| Density | 3.11 g/cm3 |
| Melting point | 310 °C (590 °F; 583 K) decomposes |
| Boiling point | 578 °C (1,072 °F; 851 K) |
| 44.28 g/100 mL (16 °C) 65.0 g/100 mL (25 °C) [1] | |
| Solubility in acetone | very slightly soluble |
| −41.0·10−6 cm3/mol | |
Refractive index (nD) | 1.524 |
| Structure [2] | |
| trigonal | |
| P31 | |
a = 10.474 Å, c = 7.443 Å | |
Lattice volume (V) | 707.2 Å3 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Oxidant |
| GHS labelling: [3] | |
  | |
| Danger | |
| H272, H315, H319, H335 | |
| P210, P220, P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P370+P378, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 4625 mg/kg (rat, oral) |
| Related compounds | |
Other anions | Rubidium sulfate Rubidium chloride |
Other cations | Lithium nitrate Sodium nitrate Potassium nitrate Caesium nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Rubidium nitrate is an inorganic compound with the formula Rb NO3. This alkali metal nitrate salt is a white hygroscopic solid that is highly soluble in water.

