 | |
| Names | |
|---|---|
| Other names titanium tetranitrate, tetranitratotitanium | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.222.601 |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Ti(NO3)4 | |
| Molar mass | 295.8866 g/mol |
| Appearance | white volatile solid |
| Density | 2.192 [3] |
| Melting point | 58 [4] °C (136 °F; 331 K) |
| Boiling point | decompose |
| Reacts [5] | |
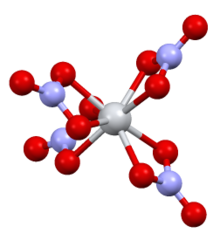
| Structure [6] | |
| monoclinic | |
| P21/C | |
a = 7.80, b = 13.57, c = 10.34 Å α = 90°, β = 125·0°, γ = 90° | |
Lattice volume (V) | 896.52 Å3 |
Formula units (Z) | 4 |
| 8 | |
| flattened tetrahedral | |
| Related compounds | |
Related compounds | hafnium nitrate, zirconium nitrate, titanium phosphate, titanium perchlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.