Related Research Articles

Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colourless and odourless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

The nitronium ion, [NO2]+, is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion [NH4]+. It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule NO2, or the protonation of nitric acid HNO3.

Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russian rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that contain only nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
An acidic oxide is an oxide that either produces an acidic solution upon addition to water, or acts as an acceptor of hydroxide ions effectively functioning as a Lewis acid. Acidic oxides will typically have a low pKa and may be inorganic or organic. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution when dissolved.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.

Manganese(VII) oxide (manganese heptoxide) is an inorganic compound with the formula Mn2O7. Manganese heptoxide is a volatile liquid with an oily consistency. It is a highly reactive and powerful oxidizer that reacts explosively with nearly any organic compound. It was first described in 1860. It is the acid anhydride of permanganic acid.

Rhenium(VII) oxide is the inorganic compound with the formula Re2O7. This yellowish solid is the anhydride of HOReO3. Perrhenic acid, Re2O7·2H2O, is closely related to Re2O7. Re2O7 is the raw material for all rhenium compounds, being the volatile fraction obtained upon roasting the host ore.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.
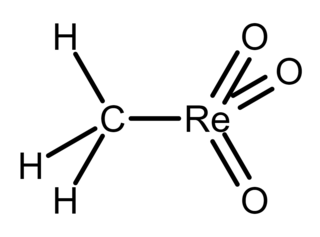
Methylrhenium trioxide, also known as methyltrioxorhenium(VII), is an organometallic compound with the formula CH3−ReO3. It is a volatile, colourless solid that has been used as a catalyst in some laboratory experiments. In this compound, rhenium has a tetrahedral coordination geometry with one methyl and three oxo ligands. The oxidation state of rhenium is +7.

Nitrogen trioxide or nitrate radical is an oxide of nitrogen with formula NO
3, consisting of three oxygen atoms covalently bound to a nitrogen atom. This highly unstable blue compound has not been isolated in pure form, but can be generated and observed as a short-lived component of gas, liquid, or solid systems.
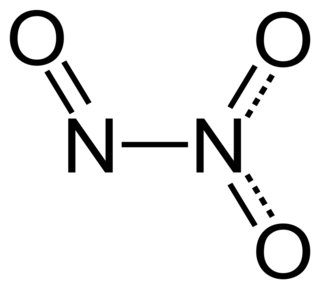
Dinitrogen trioxide is the inorganic compound with the formula N2O3. It is a nitrogen oxide. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):

Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.

Vanadyl nitrate, also called vanadium oxytrinitrate or vanadium oxynitrate is an inorganic compound of vanadium in the +5 oxidation state with nitrate ligands and oxygen. The formula is VO(NO3)3. It is a pale yellow viscous liquid.

Zirconium nitrate is a volatile anhydrous transition metal nitrate salt of zirconium with formula Zr(NO3)4. It has alternate names of zirconium tetranitrate, or zirconium(IV) nitrate.

Nitratoauric acid, hydrogen tetranitratoaurate, or simply called gold(III) nitrate is a crystalline gold compound that forms the trihydrate, HAu(NO3)4·3H2O or more correctly H5O2Au(NO3)4·H2O. This compound is an intermediate in the process of extracting gold. In older literature it is also known as aurinitric acid.

Tin(IV) nitrate is a salt of tin with nitric acid. It is a volatile white solid, subliming at 40 °C under a vacuum. Unlike other nitrates, it reacts with water to produce nitrogen dioxide.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.

Rhenium trioxide chloride is an inorganic compound with the formula ReO3Cl. It is a colorless, distillable, diamagnetic liquid. It is a rhenium oxychloride. The material is used as a reagent in the preparation of rhenium compounds.

Nitrosyl perchlorate is the inorganic compound with the formula NO(ClO4). A hygroscopic white solid, it is the salt of the nitrosonium cation with the perchlorate anion. It is an oxidant and strong electrophile, but has fallen out of use with the availability of the closely related salt nitrosonium tetrafluoroborate NO(BF4).
References
- 1 2 3 4 5 6 7 8 C. C. Addison; R. Davis; N. Logan (1967). "Rhenium trioxide nitrate". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1449–1451. doi:10.1039/J19670001449.
- 1 2 P. Scharff; E. Stumpp; M. Höhne; Y. X. Wang (1991). "Upon the intercalation of rhenium heptoxide and rhenium trioxide nitrate into graphite". Carbon. 29 (4–5): 595–597. Bibcode:1991Carbo..29..595S. doi:10.1016/0008-6223(91)90125-3.
- ↑ Romão, Carlos C.; Kühn, Fritz E.; Herrmann, Wolfgang A. (1997). "Rhenium(VII) Oxo and Imido Complexes: Synthesis, Structures, and Applications". Chemical Reviews. 97 (8): 3197–3246. doi:10.1021/cr9703212. PMID 11851489.