
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. It has the third-highest melting point and second-highest boiling point of any element at 5869 K. It resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. It shows in its compounds a wide variety of oxidation states ranging from −1 to +7.

Technetium is a chemical element; it has symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, the most common source, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only.

Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This group lies in the d-block of the periodic table, and are hence transition metals. This group is sometimes called the manganese group or manganese family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.
Refractory metals are a class of metals that are extraordinarily resistant to heat and wear. The expression is mostly used in the context of materials science, metallurgy and engineering. The definition of which elements belong to this group differs. The most common definition includes five elements: two of the fifth period and three of the sixth period. They all share some properties, including a melting point above 2000 °C and high hardness at room temperature. They are chemically inert and have a relatively high density. Their high melting points make powder metallurgy the method of choice for fabricating components from these metals. Some of their applications include tools to work metals at high temperatures, wire filaments, casting molds, and chemical reaction vessels in corrosive environments. Partly due to the high melting point, refractory metals are stable against creep deformation to very high temperatures.
In chemistry, bond energy (BE), also called the mean bond enthalpy or average bond enthalpy is a measure of bond strength in a chemical bond. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy for all bonds of the same type within the same chemical species.
Naturally occurring rhenium (75Re) is 37.4% 185Re, which is stable (although it is predicted to decay), and 62.6% 187Re, which is unstable but has a very long half-life (4.12×1010 years). Among elements with a known stable isotope, only indium and tellurium similarly occur with a stable isotope in lower abundance than the long-lived radioactive isotope.
Indium (49In) consists of two primordial nuclides, with the most common (~ 95.7%) nuclide (115In) being measurably though weakly radioactive. Its spin-forbidden decay has a half-life of 4.41×1014 years, much longer than the currently accepted age of the Universe.

Aluminium fluoride is an inorganic compound with the formula AlF3. It forms hydrates AlF3·xH2O. Anhydrous AlF3 and its hydrates are all colorless solids. Anhydrous AlF3 is used in the production of aluminium metal. Several occur as minerals.

Rhenium(VII) oxide is the inorganic compound with the formula Re2O7. This yellowish solid is the anhydride of HOReO3. Perrhenic acid, Re2O7·2H2O, is closely related to Re2O7. Re2O7 is the raw material for all rhenium compounds, being the volatile fraction obtained upon roasting the host ore.
Rhenium trioxide or rhenium(VI) oxide is an inorganic compound with the formula ReO3. It is a red solid with a metallic lustre that resembles copper in appearance. It is the only stable trioxide of the Group 7 elements (Mn, Tc, Re).
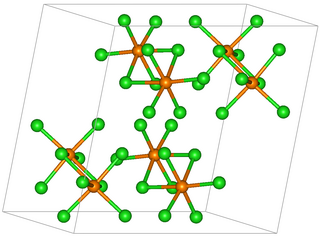
Rhenium pentachloride is an inorganic compound of chlorine and rhenium. The compound has the formula Re2Cl10 but it is usually referred to as rhenium pentachloride. It is a red-brown solid.

Potassium nonahydridorhenate(VII) is an inorganic compound having the formula K2[ReH9]. This colourless salt is soluble in water but only poorly soluble in most alcohols. This salt contains the nonahydridorhenate(VII) anion, [ReH9]2−, which is a rare example of a coordination complex bearing only hydride ligands.

Rhenium heptafluoride is the compound with the formula ReF7. It is a yellow low melting solid and is the only thermally stable metal heptafluoride. It has a distorted pentagonal bipyramidal structure similar to IF7, which was confirmed by neutron diffraction at 1.5 K. The structure is non-rigid, as evidenced by electron diffraction studies.

Rhenium(IV) oxide or rhenium dioxide is the inorganic compound with the formula ReO2. This gray to black crystalline solid is a laboratory reagent that can be used as a catalyst. It adopts the rutile structure.

Rhenium hexafluoride, also rhenium(VI) fluoride, (ReF6) is a compound of rhenium and fluorine and one of the seventeen known binary hexafluorides.

Osmium hexafluoride, also osmium(VI) fluoride, (OsF6) is a compound of osmium and fluorine, and one of the seventeen known binary hexafluorides.

Rhenium diselenide is an inorganic compound with the formula ReSe2. It has a layered structure where atoms are strongly bonded within each layer. The layers are held together by weak Van der Waals bonds, and can be easily peeled off from the bulk material.
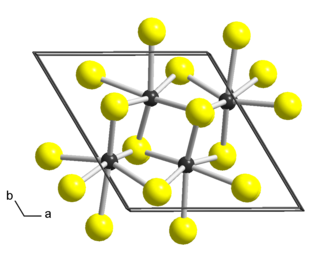
Rhenium disulfide is an inorganic compound of rhenium and sulfur with the formula ReS2. It has a layered structure where atoms are strongly bonded within each layer. The layers are held together by weak Van der Waals bonds, and can be easily peeled off from the bulk material.
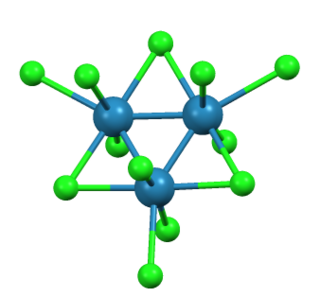
Rhenium(III) bromide is a chemical compound with the formula Re3Br9. It is a black lustrous crystalline solid. This compound reacts with water to form rhenium(IV) oxide and is isostructural with rhenium(III) chloride.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.













