
Beryllium is a chemical element; it has symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form minerals. Gemstones high in beryllium include beryl and chrysoberyl. It is a relatively rare element in the universe, usually occurring as a product of the spallation of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into heavier elements. Beryllium constitutes about 0.0004 percent by mass of Earth's crust. The world's annual beryllium production of 220 tons is usually manufactured by extraction from the mineral beryl, a difficult process because beryllium bonds strongly to oxygen.

Cadmium telluride (CdTe) is a stable crystalline compound formed from cadmium and tellurium. It is mainly used as the semiconducting material in cadmium telluride photovoltaics and an infrared optical window. It is usually sandwiched with cadmium sulfide to form a p–n junction solar PV cell.

Beryllium fluoride is the inorganic compound with the formula BeF2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.

Beryllium oxide (BeO), also known as beryllia, is an inorganic compound with the formula BeO. This colourless solid is an electrical insulator with a higher thermal conductivity than any other non-metal except diamond, and exceeds that of most metals. As an amorphous solid, beryllium oxide is white. Its high melting point leads to its use as a refractory material. It occurs in nature as the mineral bromellite. Historically and in materials science, beryllium oxide was called glucina or glucinium oxide, owing to its sweet taste.

Beryllium nitride, Be3N2, is a nitride of beryllium. It can be prepared from the elements at high temperature (1100–1500 °C); unlike beryllium azide or BeN6, it decomposes in vacuum into beryllium and nitrogen. It is readily hydrolysed forming beryllium hydroxide and ammonia. It has two polymorphic forms cubic α-Be3N2 with a defect anti-fluorite structure, and hexagonal β-Be3N2. It reacts with silicon nitride, Si3N4 in a stream of ammonia at 1800–1900 °C to form BeSiN2.

Beryllium hydroxide, Be(OH)2, is an amphoteric hydroxide, dissolving in both acids and alkalis. Industrially, it is produced as a by-product in the extraction of beryllium metal from the ores beryl and bertrandite. The natural pure beryllium hydroxide is rare (in form of the mineral behoite, orthorhombic) or very rare (clinobehoite, monoclinic). When alkali is added to beryllium salt solutions the α-form (a gel) is formed. If this left to stand or boiled, the rhombic β-form precipitates. This has the same structure as zinc hydroxide, Zn(OH)2, with tetrahedral beryllium centers.

Basic beryllium acetate is the chemical compound with the formula Be4O(O2CCH3)6. This compound adopts a distinctive structure, but it has no applications and has been only lightly studied. It is a colourless solid that is soluble in organic solvents.
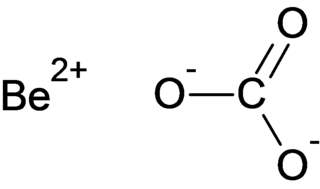
Beryllium carbonate is a chemical compound with the chemical formula BeCO3.

Beryllium bromide is the chemical compound with the formula BeBr2. It is very hygroscopic and dissolves well in water. The Be2+ cation, which is relevant to BeBr2, is characterized by the highest known charge density (Z/r = 6.45), making it one of the hardest cations and a very strong Lewis acid.

Beryllium iodide is an inorganic compound with the chemical formula BeI2. It is a hygroscopic white solid. The Be2+ cation, which is relevant to salt-like BeI2, is characterized by the highest known charge density (Z/r = 6.45), making it one of the hardest cations and a very strong Lewis acid.

Beryllium hydride is an inorganic compound with the chemical formula n. This alkaline earth hydride is a colourless solid that is insoluble in solvents that do not decompose it. Unlike the ionically bonded hydrides of the heavier Group 2 elements, beryllium hydride is covalently bonded.

Beryllium carbide, or Be2C, is a metal carbide. Similar to diamond, it is a very hard compound. It is used in nuclear reactors as a core material.

Beryllium sulfate normally encountered as the tetrahydrate, [Be(H2O)4]SO4 is a white crystalline solid. It was first isolated in 1815 by Jons Jakob Berzelius. Beryllium sulfate may be prepared by treating an aqueous solution of many beryllium salts with sulfuric acid, followed by evaporation of the solution and crystallization. The hydrated product may be converted to anhydrous salt by heating at 400 °C.
Acute beryllium poisoning is acute chemical pneumonitis resulting from the toxic effect of beryllium in its elemental form or in various chemical compounds, and is distinct from berylliosis. After occupational safety procedures were put into place following the realization that the metal caused berylliosis around 1950, acute beryllium poisoning became extremely rare.

Beryllium nitrate is an inorganic compound with the idealized chemical formula Be(NO3)2. The formula suggests a salt, but, as for many beryllium compounds, the compound is highly covalent. Little of its chemistry is well known. "When added to water, brown fumes are evolved; when hydrolyzed in sodium hydroxide solution, both nitrate and nitrite ions are produced."

Beryllium azide, Be(N3)2, is an inorganic compound. It is the beryllium analog of hydrazoic acid.
Beryllium monohydride (BeH) is an example of a molecule with a half-bond order according to molecular orbital theory. It is a metastable monoradical species which has only been observed in the gas phase. In beryllium monohydride, beryllium has a valency of one, and hydrogen has a valency of one.

Beryllium borohydride is an inorganic compound with the chemical formula Be[BH4]2.
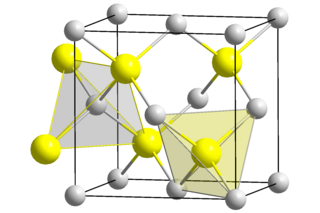
Beryllium sulfide (BeS) is an ionic compound from the sulfide group with the formula BeS. It is a white solid with a sphalerite structure that is decomposed by water and acids.
Lithium telluride (Li2Te) is an inorganic compound of lithium and tellurium. Along with LiTe3, it is one of the two intermediate solid phases in the lithium-tellurium system. It can be prepared by directly reacting lithium and tellurium in a beryllium oxide crucible at 950°C.

















