Related Research Articles

The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.

Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.
A period 5 element is one of the chemical elements in the fifth row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that elements with similar behaviour fall into the same vertical columns. The fifth period contains 18 elements, beginning with rubidium and ending with xenon. As a rule, period 5 elements fill their 5s shells first, then their 4d, and 5p shells, in that order; however, there are exceptions, such as rhodium.
The Goldschmidt classification, developed by Victor Goldschmidt (1888–1947), is a geochemical classification which groups the chemical elements within the Earth according to their preferred host phases into lithophile (rock-loving), siderophile (iron-loving), chalcophile, and atmophile (gas-loving) or volatile.

Calaverite, or gold telluride, is an uncommon telluride of gold, a metallic mineral with the chemical formula AuTe2, with approximately 3% of the gold replaced by silver. It was first discovered in Calaveras County, California in 1861, and was named for the county in 1868.

Sylvanite or silver gold telluride, chemical formula (Ag,Au)Te2, is the most common telluride of gold.
The telluride ion is the anion Te2− and its derivatives. It is analogous to the other chalcogenide anions, the lighter O2−, S2−, and Se2−, and the heavier Po2−.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.
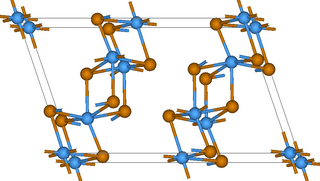
Tantalum telluride (TaTe2) is a chemical compound of tantalum and tellurium. Tantalum also forms a tantalum rich telluride with the approximate formula Ta1.6Te that is unusual in that it forms dodecagonal chalcogenide quasicrystals, a formation that cannot occur in a normal crystal because it does not result in a periodic crystal lattice.

Hydrogen telluride is the inorganic compound with the formula H2Te. A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas. Although unstable in ambient air, the gas can exist at very low concentrations long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations. Most compounds with Te–H bonds (tellurols) are unstable with respect to loss of H2. H2Te is chemically and structurally similar to hydrogen selenide, both are acidic. The H–Te–H angle is about 90°. Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.

Gold compounds are compounds by the element gold (Au). Although gold is the most noble of the noble metals, it still forms many diverse compounds. The oxidation state of gold in its compounds ranges from −1 to +5, but Au(I) and Au(III) dominate its chemistry. Au(I), referred to as the aurous ion, is the most common oxidation state with soft ligands such as thioethers, thiolates, and organophosphines. Au(I) compounds are typically linear. A good example is Au(CN)−2, which is the soluble form of gold encountered in mining. The binary gold halides, such as AuCl, form zigzag polymeric chains, again featuring linear coordination at Au. Most drugs based on gold are Au(I) derivatives.
The indium chalcogenides include all compounds of indium with the chalcogen elements, oxygen, sulfur, selenium and tellurium. (Polonium is excluded as little is known about its compounds with indium). The best-characterised compounds are the In(III) and In(II) chalcogenides e.g. the sulfides In2S3 and InS.
This group of compounds has attracted a lot of research attention because they include semiconductors, photovoltaics and phase-change materials. In many applications indium chalcogenides are used as the basis of ternary and quaternary compounds such as indium tin oxide, ITO and copper indium gallium selenide, CIGS.

Coloradoite, also known as mercury telluride (HgTe), is a rare telluride ore associated with metallic deposit. Gold usually occurs within tellurides, such as coloradoite, as a high-finess native metal.

Molybdenum(IV) telluride, molybdenum ditelluride or just molybdenum telluride is a compound of molybdenum and tellurium with formula MoTe2, corresponding to a mass percentage of 27.32% molybdenum and 72.68% tellurium.
Tellurium compounds are compounds containing the element tellurium (Te). Tellurium belongs to the chalcogen family of elements on the periodic table, which also includes oxygen, sulfur, selenium and polonium: Tellurium and selenium compounds are similar. Tellurium exhibits the oxidation states −2, +2, +4 and +6, with +4 being most common.
Copper telluride may refer to:
The telluride bromides are chemical compounds that contain both telluride ions (Te2−) and bromide ions (Br−). They are in the class of mixed anion compounds or chalcogenide halides.

The gold cycle is the biogeochemical cycling of gold through the lithosphere, hydrosphere, atmosphere, and biosphere. Gold is a noble transition metal that is highly mobile in the environment and subject to biogeochemical cycling, driven largely by microorganisms. Gold undergoes processes of solubilization, stabilization, bioreduction, biomineralization, aggregation, and ligand utilization throughout its cycle. These processes are influenced by various microbial populations and cycling of other elements such as carbon, nitrogen, and sulfur. Gold exists in several forms in the Earth's surface environment including Au(I/III)-complexes, nanoparticles, and placer gold particles. The gold biogeochemical cycle is highly complex and strongly intertwined with cycling of other metals including silver, copper, iron, manganese, arsenic, and mercury. Gold is important in the biotech field for applications such as mineral exploration, processing and remediation, development of biosensors and drug delivery systems, industrial catalysts, and for recovery of gold from electronic waste.
Tellurogallates are chemical compounds which contain anionic units of tellurium connected to gallium. They can be considered as gallates where tellurium substitutes for oxygen. Similar compounds include the thiogallates, selenogallates, telluroaluminates, telluroindates and thiostannates. They are in the category of chalcogenotrielates or more broadly tellurometallates or chalcogenometallates.
References
- ↑ Luo, H.L.; Merriam, M.F.; Hamilton, D.C. (1964). "Superconducting Metastable Compounds". Science. 145 (3632): 581–583. Bibcode:1964Sci...145..581L. doi:10.1126/science.145.3632.581. PMID 17735806. S2CID 41529555.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.