
Holmium is a chemical element; it has symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like many other lanthanides, holmium is too reactive to be found in native form, as pure holmium slowly forms a yellowish oxide coating when exposed to air. When isolated, holmium is relatively stable in dry air at room temperature. However, it reacts with water and corrodes readily, and also burns in air when heated.

Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.
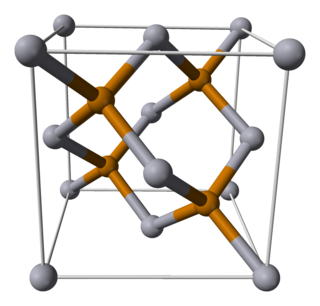
Mercury telluride (HgTe) is a binary chemical compound of mercury and tellurium. It is a semi-metal related to the II-VI group of semiconductor materials. Alternative names are mercuric telluride and mercury(II) telluride.
Rubidium telluride is the inorganic compound with the formula Rb2Te. It is a yellow-green powder that melts at either 775 °C or 880 °C (two different values have been reported). It is an obscure material of minor academic interest.
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.
Indium(III) selenide is a compound of indium and selenium. It has potential for use in photovoltaic devices and has been the subject of extensive research. The two most common phases, α and β, have a layered structure, while γ has a "defect wurtzite structure." In all, five polymorphs are known: α, β, γ, δ, κ. The α-β phase transition is accompanied by a change in electrical conductivity. The band gap of γ-In2Se3 is approximately 1.9 eV.
Tellurium compounds are compounds containing the element tellurium (Te). Tellurium belongs to the chalcogen family of elements on the periodic table, which also includes oxygen, sulfur, selenium and polonium: Tellurium and selenium compounds are similar. Tellurium exhibits the oxidation states −2, +2, +4 and +6, with +4 being most common.
Holmium(III) fluoride is an inorganic compound with a chemical formula of HoF3.

Indium(III) iodide or indium triiodide is a chemical compound of indium and iodine with the formula InI3.
Lithium telluride (Li2Te) is an inorganic compound of lithium and tellurium. Along with LiTe3, it is one of the two intermediate solid phases in the lithium-tellurium system. It can be prepared by directly reacting lithium and tellurium in a beryllium oxide crucible at 950°C.
Tellurogallates are chemical compounds which contain anionic units of tellurium connected to gallium. They can be considered as gallates where tellurium substitutes for oxygen. Similar compounds include the thiogallates, selenogallates, telluroaluminates, telluroindates and thiostannates. They are in the category of chalcogenotrielates or more broadly tellurometallates or chalcogenometallates.

Holmium acetate is the acetate salt of holmium, with a chemical formula of Ho(CH3COO)3.
Tellurogermanates or telluridogermanates are compounds with anions with tellurium bound to germanium. They are analogous with germanates, thiogermanates and selenidogermanates.
Lithium tritelluride is an intercalary compound of lithium and tellurium with empirical formula LiTe
3. It is one of three known members of the Li-Te system, the others being the raw metals and lithium telluride.

Holmium acetylacetonate is a coordination complex, with the chemical formula of Ho(C5H7O2)3 or Ho(acac)3. It can be obtained via the reaction between metallic holmium or holmium(III) hydride with acetylacetone, or via the reaction between holmium(III) chloride and ammonium acetylacetonate. Its anhydrous form is stable in a dry atmosphere but forms a hydrate in humid air.

Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.
Holmium(III) selenide is an inorganic compound with the chemical formula Ho2Se3.
Thulium(III) telluride is an inorganic compound, one of the tellurides of thulium, with the chemical formula Tm2Te3. It is an orthorhombic crystal with space group Fddd. It can dissolve in lead telluride at high temperatures to form a solid solution.
Ytterbium monotelluride is an inorganic compound, one of the tellurides of ytterbium, with the chemical formula YbTe.






