The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.

Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.

Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides.

Europium(III) chloride is an inorganic compound with the formula EuCl3. The anhydrous compound is a yellow solid. Being hygroscopic it rapidly absorbs water to form a white crystalline hexahydrate, EuCl3·6H2O, which is colourless. The compound is used in research.

Manganese(II) chloride is the dichloride salt of manganese, MnCl2. This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl2·2H2O) and tetrahydrate (MnCl2·4H2O), with the tetrahydrate being the most common form. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d5 configurations.
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.
The telluride ion is the anion Te2− and its derivatives. It is analogous to the other chalcogenide anions, the lighter O2−, S2−, and Se2−, and the heavier Po2−.

Chromium(II) chloride describes inorganic compounds with the formula CrCl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue air-sensitive solutions of the tetrahydrate Cr(H2O)4Cl2. Chromium(II) chloride has no commercial uses but is used on a laboratory-scale for the synthesis of other chromium complexes.

Tellurium tetrachloride is the inorganic compound with the empirical formula TeCl4. The compound is volatile, subliming at 200 °C at 0.1 mmHg. Molten TeCl4 is ionic, dissociating into TeCl3+ and Te2Cl102−.

Ytterbium(III) chloride (YbCl3) is an inorganic chemical compound. It reacts with NiCl2 to form a very effective catalyst for the reductive dehalogenation of aryl halides. It is poisonous if injected, and mildly toxic by ingestion. It is an experimental teratogen, known to irritate the skin and eyes.
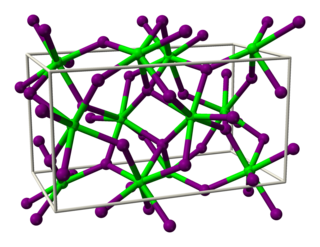
Ytterbium(II) chloride (YbCl2) is an inorganic chemical compound. It was first prepared in 1929 by W. K. Klemm and W. Schuth, by reduction of ytterbium(III) chloride, YbCl3, using hydrogen.
Organotellurium chemistry describes the synthesis and properties of organotellurium compounds, chemical compounds containing a carbon-tellurium chemical bond. Organotellurium chemistry is a lightly studied area, in part because of it having few applications.
Tellurium compounds are compounds containing the element tellurium (Te). Tellurium belongs to the chalcogen family of elements on the periodic table, which also includes oxygen, sulfur, selenium and polonium: Tellurium and selenium compounds are similar. Tellurium exhibits the oxidation states −2, +2, +4 and +6, with +4 being most common.

Europium(II) chloride is an inorganic compound with a chemical formula EuCl2. When it is irradiated by ultraviolet light, it has bright blue fluorescence.

Transition metal acyl complexes describes organometallic complexes containing one or more acyl (RCO) ligands. Such compounds occur as transient intermediates in many industrially useful reactions, especially carbonylations.
Lanthanide trichlorides are a family of inorganic compound with the formula LnCl3, where Ln stands for a lanthanide metal. The trichlorides are standard reagents in applied and academic chemistry of the lanthanides. They exist as anhydrous solids and as hydrates.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Ytterbium compounds are chemical compounds that contain the element ytterbium (Yb). The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium, and thulium, the trihalides of ytterbium can be reduced to the dihalides by hydrogen, zinc dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide (CaO).
Lanthanide chlorides are a group of chemical compounds that can form between a lanthanide element and chlorine. The lanthanides in these compounds are usually in the +2 and +3 oxidation states, although compounds with lanthanides in lower oxidation states exist.










