
Erbium is a chemical element; it has symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare-earth element, originally found in the gadolinite mine in Ytterby, Sweden, which is the source of the element's name.

Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).
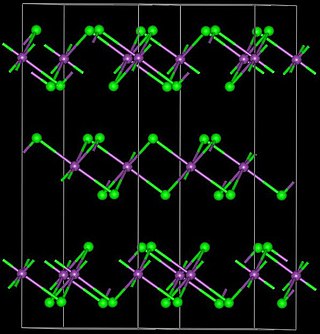
Yttrium(III) bromide is an inorganic compound with the chemical formula YBr3. It is a white solid. Anhydrous yttrium(III) bromide can be produced by reacting yttrium oxide or yttrium(III) bromide hydrate and ammonium bromide. The reaction proceeds via the intermediate (NH4)3YBr6. Another method is to react yttrium carbide (YC2) and elemental bromine. Yttrium(III) bromide can be reduced by yttrium metal to YBr or Y2Br3. It can react with osmium to produce Y4Br4Os.

Ytterbium(III) chloride (YbCl3) is an inorganic chemical compound. It reacts with NiCl2 to form a very effective catalyst for the reductive dehalogenation of aryl halides. It is poisonous if injected, and mildly toxic by ingestion. It is an experimental teratogen, known to irritate the skin and eyes.

Terbium(III) bromide (TbBr3) is a crystalline chemical compound.
Scandium bromide, or ScBr3, is a trihalide, hygroscopic, water-soluble chemical compound of scandium and bromine.

Neodymium(III) bromide is an inorganic salt of bromine and neodymium the formula NdBr3. The anhydrous compound is an off-white to pale green solid at room temperature, with an orthorhombic PuBr3-type crystal structure. The material is hygroscopic and forms a hexahydrate in water (NdBr3· 6H2O), similar to the related neodymium(III) chloride.
Samarium(III) bromide is a crystalline compound of one samarium and three bromine atoms with the chemical formula of SmBr3. Samarium(III) bromide is a dark brown powder at room temperature. The compound has a crystal structure isotypic to that of plutonium(III) bromide.
Curium(III) bromide is the bromide salt of curium. It has an orthorhombic crystal structure.
Lanthanide trichlorides are a family of inorganic compound with the formula LnCl3, where Ln stands for a lanthanide metal. The trichlorides are standard reagents in applied and academic chemistry of the lanthanides. They exist as anhydrous solids and as hydrates.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Dysprosium(III) bromide is an inorganic compound of bromine and dysprosium, with the chemical formula of DyBr3.

Promethium(III) bromide is an inorganic compound, with the chemical formula of PmBr3. It is radioactive salt. It is a crystal of the hexagonal crystal system, with the space group of P63/mc (No. 176).
Erbium compounds are compounds containing the element erbium (Er). These compounds are usually dominated by erbium in the +3 oxidation state, although the +2, +1 and 0 oxidation states have also been reported.
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.
Actinium compounds are compounds containing the element actinium (Ac). Due to actinium's intense radioactivity, only a limited number of actinium compounds are known. These include: AcF3, AcCl3, AcBr3, AcOF, AcOCl, AcOBr, Ac2S3, Ac2O3, AcPO4 and Ac(NO3)3. Except for AcPO4, they are all similar to the corresponding lanthanum compounds. They all contain actinium in the oxidation state +3. In particular, the lattice constants of the analogous lanthanum and actinium compounds differ by only a few percent.

Ytterbium(II) iodide is an iodide of ytterbium, with the chemical formula of YbI2. It is a yellow solid.

Ytterbium(III) iodide is one of ytterbium's iodides, with the chemical formula of YbI3.










