 | |
 Anhydrous | |
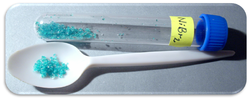 Hexahydrate | |
| Names | |
|---|---|
| IUPAC name Nickel(II) bromide | |
| Other names Nickel dibromide, Nickel bromide, Nickelous bromide | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.033.318 |
| EC Number |
|
PubChem CID | |
| UNII | |
| UN number | 3288 (NICKEL BROMIDE) |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| NiBr2 | |
| Molar mass | 218.53 g/mol |
| Appearance | yellow-brown crystals |
| Odor | odorless |
| Density | 5.10 g/cm3 [1] |
| Melting point | 963 °C (1,765 °F; 1,236 K) sublimes [1] |
| 1.13 kg/L (0 °C) 1.22 kg/L (10 °C) 1.31 kg/L (20 °C) [1] 1.44 kg/L (40 °C) 1.55 kg/L (100 °C) [2] | |
| Band gap | 2.5 eV [3] |
| +5600.0·10−6 cm3/mol [4] | |
| Structure [5] | |
| hexagonal, hR9 | |
| R3m, No. 166 | |
a = 0.36998 nm, c = 1.82796 nm | |
Formula units (Z) | 3 |
| Thermochemistry [6] | |
Std enthalpy of formation (ΔfH⦵298) | −212.1 kJ·mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Irritant, corrosive |
| GHS labelling: [7] | |
  | |
| Danger | |
| H317, H334, H341, H350i, H360D, H372, H410 | |
| P203, P233, P260, P264, P270, P271, P272, P273, P280, P284, P302+P352, P304+P340, P318, P319, P321, P333+P317, P342+P316, P362+P364, P391, P403, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions | nickel(II) fluoride nickel(II) chloride nickel(II) iodide |
Other cations | cobalt(II) bromide copper(II) bromide palladium(II) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Nickel(II) bromide is the name for the inorganic compounds with the chemical formula NiBr2(H2O)x. The value of x can be 0 for the anhydrous material, as well as 2, 3, or 6 for the three known hydrate forms. The anhydrous material is a yellow-brown solid which dissolves in water to give blue-green hexahydrate (see picture).

