
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen or moisture slowly to form a black coating. Gadolinium below its Curie point of 20 °C (68 °F) is ferromagnetic, with an attraction to a magnetic field higher than that of nickel. Above this temperature it is the most paramagnetic element. It is found in nature only in an oxidized form. When separated, it usually has impurities of the other rare-earths because of their similar chemical properties.

Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with water, evolving hydrogen gas. Terbium is never found in nature as a free element, but it is contained in many minerals, including cerite, gadolinite, monazite, xenotime and euxenite.

Gadolinium(III) chloride, also known as gadolinium trichloride, is GdCl3. It is a colorless, hygroscopic, water-soluble solid. The hexahydrate GdCl3∙6H2O is commonly encountered and is sometimes also called gadolinium trichloride. Gd3+ species are of special interest because the ion has the maximum number of unpaired spins possible, at least for known elements. With seven valence electrons and seven available f-orbitals, all seven electrons are unpaired and symmetrically arranged around the metal. The high magnetism and high symmetry combine to make Gd3+ a useful component in NMR spectroscopy and MRI.
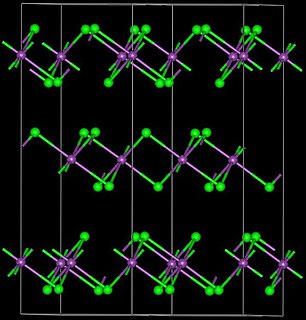
Yttrium(III) bromide is an inorganic compound with the chemical formula YBr3. It is a white solid. Anhydrous yttrium(III) bromide can be produced by reacting yttrium oxide or yttrium(III) bromide hydrate and ammonium bromide. The reaction proceeds via the intermediate (NH4)3YBr6. Another method is to react yttrium carbide (YC2) and elemental bromine. Yttrium(III) bromide can be reduced by yttrium metal to YBr or Y2Br3. It can react with osmium to produce Y4Br4Os.
Gadolinium oxysulfide (Gd2O2S), also called gadolinium sulfoxylate, GOS or Gadox, is an inorganic compound, a mixed oxide-sulfide of gadolinium.
MRI contrast agents are contrast agents used to improve the visibility of internal body structures in magnetic resonance imaging (MRI). The most commonly used compounds for contrast enhancement are gadolinium-based. Such MRI contrast agents shorten the relaxation times of nuclei within body tissues following oral or intravenous administration.

Neodymium(III) bromide is an inorganic salt of bromine and neodymium the formula NdBr3. The anhydrous compound is an off-white to pale green solid at room temperature, with an orthorhombic PuBr3-type crystal structure. The material is hydroscopic and forms a hexahydrate in water (NdBr3· 6H2O), similar to the related neodymium(III) chloride.
Samarium(III) bromide is a crystalline compound of one samarium and three bromine atoms with the chemical formula of SmBr3. Samarium(III) bromide is a dark brown powder at room temperature. The compound has a crystal structure isotypic to that of plutonium(III) bromide.

Europium(II) bromide is a crystalline compound of one europium atom and two bromine atoms. Europium(II) bromide is a white powder at room temperature, and odorless. Europium dibromide is hygroscopic.
Europium(III) bromide is a crystalline compound, a salt, made of one europium and three bromine atoms. Europium tribromide is a grey powder at room temperature. It is odorless. Europium tribromide is hygroscopic.
Holmium(III) bromide is a crystalline compound made of one holmium atom and three bromine atoms. Holmium bromide is a yellow powder at room temperature. Holmium bromide is hygroscopic. Holmium bromide is odorless.
Thulium(III) bromide is a crystalline compound of one thulium atom and three bromine atoms. The salt is a white powder at room temperature. It is hygroscopic.
Gadolinium(III) fluoride is an inorganic compound with a chemical formula GdF3.
Carbide chlorides are mixed anion compounds containing chloride anions and anions consisting entirely of carbon. In these compounds there is no bond between chlorine and carbon. But there is a bond between a metal and carbon. Many of these compounds are cluster compounds, in which metal atoms encase a carbon core, with chlorine atoms surrounding the cluster. The chlorine may be shared between clusters to form polymers or layers. Most carbon chloride compounds contain rare earth elements. Some are known from group 4 elements. The hexatungsten carbon cluster can be oxidised and reduced, and so have different numbers of chlorine atoms included.
Carbide bromides are mixed anion compounds containing bromide and carbide anions. Many carbide bromides are cluster compounds, containing on, two or more carbon atoms in a core, surrounded by a layer of metal atoms, encased in a shell of bromide ions. These ions may be shared between clusters to form chains, double chains or layers.

Dysprosium(III) bromide is an inorganic compound of bromine and dysprosium, with the chemical formula of DyBr3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Gadolinium(III) iodide is an iodide of gadolinium, with the chemical formula of GdI3. It is a yellow, highly hygroscopic solid with a bismuth(III) iodide-type crystal structure. In air, it quickly absorbs moisture and forms hydrates. The corresponding oxide iodide is also readily formed at elevated temperature.

Gadolinium diiodide is an inorganic compound, with the chemical formula of GdI2. It is an electride, with the ionic formula of Gd3+(I−)2e−, and therefore not a true gadolinium(II) compound. It is ferromagnetic at 276 K with a saturation magnetization of 7.3 B; it exhibits a large negative magnetoresistance (~70%) at 7 T near room temperature. It can be obtained by reacting gadolinium and gadolinium(III) iodide at a high temperature:

Promethium(III) bromide is an inorganic compound, with the chemical formula of PmBr3. It is radioactive. It is a crystal of the hexagonal crystal system, with the space group of P63/mc (No. 176).









