The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.

Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and forms a dark blue solution in THF. It is a strong one-electron reducing agent that is used in organic synthesis.

Cerium(III) chloride (CeCl3), also known as cerous chloride or cerium trichloride, is a compound of cerium and chlorine. It is a white hygroscopic salt; it rapidly absorbs water on exposure to moist air to form a hydrate, which appears to be of variable composition, though the heptahydrate CeCl3·7H2O is known. It is highly soluble in water, and (when anhydrous) it is soluble in ethanol and acetone.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:

Mercury(II) iodide is a chemical compound with the molecular formula HgI2. It is typically produced synthetically but can also be found in nature as the extremely rare mineral coccinite. Unlike the related mercury(II) chloride it is hardly soluble in water (<100 ppm).
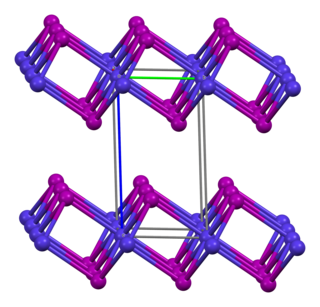
Cobalt(II) iodide or cobaltous iodide are the inorganic compounds with the formula CoI2 and the hexahydrate CoI2(H2O)6. These salts are the principal iodides of cobalt.

Reductions with samarium(II) iodide involve the conversion of various classes of organic compounds into reduced products through the action of samarium(II) iodide, a mild one-electron reducing agent.

Few compounds of californium have been made and studied. The only californium ion that is stable in aqueous solutions is the californium(III) cation. The other two oxidation states are IV (strong oxidizing agents) and II (strong reducing agents). The element forms a water-soluble chloride, nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate or hydroxide. If problems of availability of the element could be overcome, then CfBr2 and CfI2 would likely be stable.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium and iodine, with the chemical formula PrI3. It forms green crystals. It is soluble in water.

Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Gadolinium diiodide is an inorganic compound, with the chemical formula of GdI2. It is an electride, with the ionic formula of Gd3+(I−)2e−, and therefore not a true gadolinium(II) compound. It is ferromagnetic at 276 K with a saturation magnetization of 7.3 B; it exhibits a large negative magnetoresistance (~70%) at 7 T near room temperature. It can be obtained by reacting gadolinium and gadolinium(III) iodide at a high temperature:

Lanthanum diiodide is an iodide of lanthanum, with the chemical formula of LaI2. It is an electride, actually having a chemical formula of La3+[(I−)2e−].
Cerium compounds are compounds containing the element cerium (Ce), a lanthanide. Cerium exists in two main oxidation states, Ce(III) and Ce(IV). This pair of adjacent oxidation states dominates several aspects of the chemistry of this element. Cerium(IV) aqueous solutions may be prepared by reacting cerium(III) solutions with the strong oxidizing agents peroxodisulfate or bismuthate. The value of E⦵(Ce4+/Ce3+) varies widely depending on conditions due to the relative ease of complexation and hydrolysis with various anions, although +1.72 V is representative. Cerium is the only lanthanide which has important aqueous and coordination chemistry in the +4 oxidation state.

Tungsten(II) iodide is an iodide of tungsten, with the chemical formula [W6I8]I4, or abbreviated as WI2.
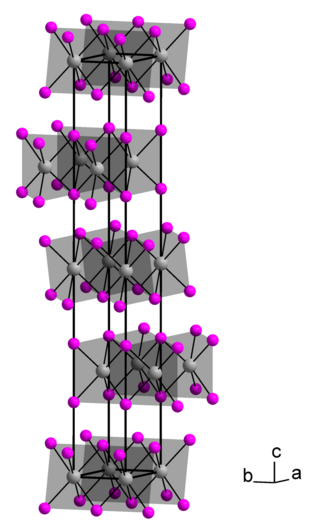
Thorium diiodide is an iodide of thorium, with the chemical formula of ThI2. It is an electride with the ionic formula Th4+(I-)2e-2. It is air-sensitive.













