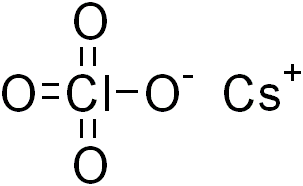Ammonium perchlorate ("AP") is an inorganic compound with the formula NH4ClO4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer. Combined with a fuel, it can be used as a rocket propellant called ammonium perchlorate composite propellant. Its instability has involved it in a number of accidents, such as the PEPCON disaster.

Perchloric acid is a mineral acid with the formula HClO4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.

A perchlorate is a chemical compound containing the perchlorate ion, ClO4-, the conjugate base of perchloric acid (ionic perchlorate). As counterions, there can be metal cations, quaternary ammonium cations or other ions, for example, nitronium cation (NO2+).

Potassium perchlorate is the inorganic salt with the chemical formula KClO4. Like other perchlorates, this salt is a strong oxidizer although it usually reacts very slowly with organic substances. This, usually obtained as a colorless, crystalline solid, is a common oxidizer used in fireworks, ammunition percussion caps, explosive primers, and is used variously in propellants, flash compositions, stars, and sparklers. It has been used as a solid rocket propellant, although in that application it has mostly been replaced by the higher performance ammonium perchlorate.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.

A permanganate is a chemical compound with the manganate(VII) ion, MnO−
4, the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition metal ion with a tetrahedral structure. Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media. The exact chemical reaction depends on the carbon-containing reactants present and the oxidant used. For example, trichloroethane (C2H3Cl3) is oxidised by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).

Sodium perchlorate is the inorganic compound with the chemical formula NaClO4. It is a white crystalline, hygroscopic solid that is highly soluble in water and in alcohol. It is usually encountered as the monohydrate. The compound is noteworthy as the most water-soluble of the common perchlorate salts.
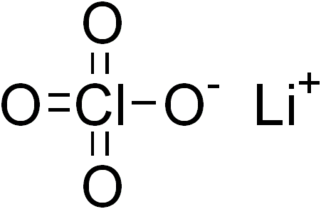
Lithium perchlorate is the inorganic compound with the formula LiClO4. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate.
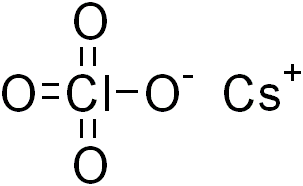
Caesium perchlorate or cesium perchlorate (CsClO4), is a perchlorate of caesium. It forms white crystals, which are sparingly soluble in cold water and ethanol. It dissolves more easily in hot water.

Sodium pertechnetate is the inorganic compound with the formula NaTcO4. This colourless salt contains the pertechnetate anion, TcO−
4. The radioactive 99m
Tc
O−
4 anion is an important radiopharmaceutical for diagnostic use. The advantages to 99m
Tc
include its short half-life of 6 hours and the low radiation exposure to the patient, which allow a patient to be injected with activities of more than 30 millicuries. Na[99m
Tc
O
4] is a precursor to a variety of derivatives that are used to image different parts of the body.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.

Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO
4F or FOClO
3. It is an extremely unstable gas that explodes spontaneously and has a penetrating odor.
Barium perchlorate is a powerful oxidizing agent, with the formula Ba(ClO4)2. It is used in the pyrotechnic industry.
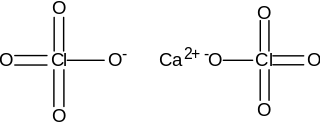
Calcium perchlorate is classified as a metal perchlorate salt with the molecular formula Ca(ClO4)2. It is an inorganic compound that is a yellow-white crystalline solid in appearance. As a strong oxidizing agent, it reacts with reducing agents when heated to generate heat and products that may be gaseous. Calcium perchlorate has been categorized as having explosive reactivity. Ca(ClO4)2 is a common chemical on the soil of planet Mars, counting for almost 1% of the Martian dust, by weight.

Titanium perchlorate is a molecular compound of titanium and perchlorate groups with formula Ti(ClO4)4. Anhydrous titanium perchlorate decomposes explosively at 130 °C and melts at 85 °C with a slight decomposition. It can sublime in a vacuum as low as 70 °C, and can form vapour at up to 120°. Titanium perchlorate is quite volatile. It has density 2.35. It decomposes to TiO2, ClO2 and dioxygen O2 Also TiO(ClO4)2 is formed during decomposition.
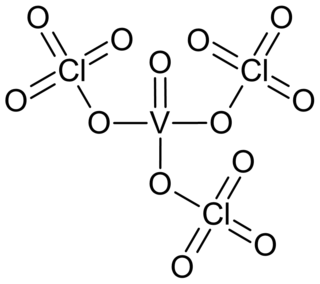
Vanadyl perchlorate or vanadyl triperchlorate is a golden yellow coloured liquid or crystalline compound of vanadium, oxygen and perchlorate group. The substance consists of molecules covalently bound and is quite volatile; it ignites organic solvents on contact and explodes at temperatures above 80 °C.

Nickel(II) perchlorate is a inorganic compound with the chemical formula of Ni(ClO4)2, and it is a strong oxidizing agent. Its colours are different depending on water. For example, the hydrate forms cyan crystals, the pentahydrate forms green crystals, but the hexahydrate (Ni(ClO4)2·6H2O) forms blue crystals.
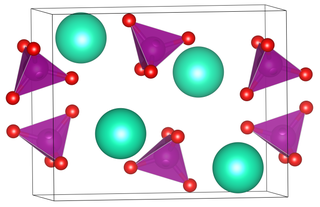
Rubidium permanganate is the permanganate salt of rubidium, with the chemical formula RbMnO
4.