
Ammonium perchlorate ("AP") is an inorganic compound with the formula NH4ClO4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer. Combined with a fuel, it can be used as a rocket propellant called ammonium perchlorate composite propellant. Its instability has involved it in a number of accidents, such as the PEPCON disaster.

Perchloric acid is a mineral acid with the formula HClO4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.

Hydrazoic acid, also known as hydrogen azide or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
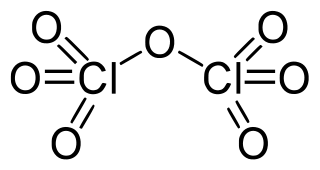
Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:

Sodium perchlorate is the inorganic compound with the chemical formula NaClO4. It is a white crystalline, hygroscopic solid that is highly soluble in water and in alcohol. It is usually encountered as the monohydrate. The compound is noteworthy as the most water-soluble of the common perchlorate salts.
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid.
Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO(OH).
A solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and anions dissolve in or precipitate from solution.

Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO
4F or FOClO
3. It is an extremely unstable gas that explodes spontaneously and has a penetrating odor.
Barium perchlorate is a powerful oxidizing agent, with the formula Ba(ClO4)2. It is used in the pyrotechnic industry.
Germanium(II) hydroxide, normally written as Ge(OH)2, is a poorly characterised compound, sometimes called hydrous germanium(II) oxide or germanous hydroxide. It was first reported by Winkler in 1886.
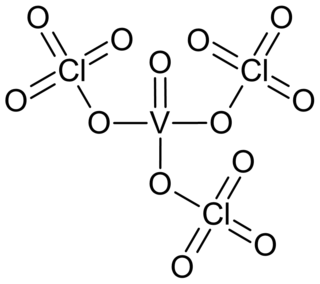
Vanadyl perchlorate or vanadyl triperchlorate is a golden yellow coloured liquid or crystalline compound of vanadium, oxygen and perchlorate group. The substance consists of molecules covalently bound and is quite volatile.

Rhodium(III) perchlorate refers to the inorganic compound with the formula Rh(H2O)6(ClO4)3. It is a hygroscopic yellow solid. It is the perchlorate salt of the tricationic aquo complex [Rh(H2O)6]3+. The compound is prepared by treating hydrated rhodium(III) chloride and perchloric acid at elevated temperatures:
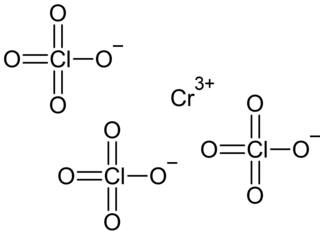
Chromium(III) perchlorate is an inorganic compound with the chemical formula Cr(ClO4)3. It's hexahydrate Cr(ClO4)3·6H2O is a cyan solid that dissolves in water.

Nickel(II) perchlorate is a inorganic compound with the chemical formula of Ni(ClO4)2, and it is a strong oxidizing agent. Its colours are different depending on water. For example, the hydrate forms cyan crystals, the pentahydrate forms green crystals, but the hexahydrate (Ni(ClO4)2·6H2O) forms blue crystals.

Neodymium(III) perchlorate is an inorganic compound. It is a salt of neodymium and perchloric acid with the chemical formula of Nd(ClO4)3 – it is soluble in water, forming purple-pink, hydrated crystals.

Lead(II) perchlorate is a chemical compound with the formula Pb(ClO4)2·xH2O, where is x is 0,1, or 3. It is an extremely hygroscopic white solid that is very soluble in water.

Zinc perchlorate is the inorganic compound with the chemical formula Zn(ClO4)2 which forms the hexahydrate.

Yttrium perchlorate is the inorganic compound with the chemical formula Y(ClO
4)
3. The compound is an yttrium salt of perchloric acid.
Ruthenium(IV) tetrachloride is an inorganic compound, a metal salt of ruthenium and hydrochloric acid with the formula RuCl4. The compound forms brown crystals, dissolves in cold water, and creates a hydrate.
















