Perchloric acid is a mineral acid with the formula HClO4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.

Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used
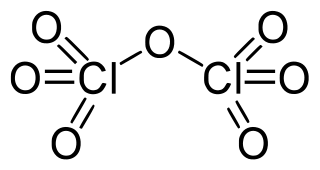
Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:

Sodium perchlorate is the inorganic compound with the chemical formula NaClO4. It is a white crystalline, hygroscopic solid that is highly soluble in water and in alcohol. It is usually encountered as the monohydrate. The compound is noteworthy as the most water-soluble of the common perchlorate salts.

Magnesium perchlorate is a powerful oxidizing agent, with the formula Mg(ClO4)2. The salt is also a superior drying agent for gas analysis.

Silver perchlorate is the chemical compound with the formula AgClO4. This white solid forms a monohydrate and is mildly deliquescent. It is a useful source of the Ag+ ion, although the presence of perchlorate presents risks. It is used as a catalyst in organic chemistry.
A solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and anions dissolve in or precipitate from solution.

Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO
4F or FOClO
3. It is an extremely unstable gas that explodes spontaneously and has a penetrating odor.
Barium perchlorate is a powerful oxidizing agent, with the formula Ba(ClO4)2. It is used in the pyrotechnic industry.
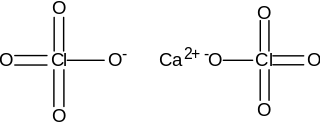
Calcium perchlorate is classified as a metal perchlorate salt with the molecular formula Ca(ClO4)2. It is an inorganic compound that is a yellow-white crystalline solid in appearance. As a strong oxidizing agent, it reacts with reducing agents when heated to generate heat and products that may be gaseous. Calcium perchlorate has been categorized as having explosive reactivity. Ca(ClO4)2 is a common chemical on the soil of planet Mars, counting for almost 1% of the Martian dust, by weight.

Titanium perchlorate is a molecular compound of titanium and perchlorate groups with formula Ti(ClO4)4. Anhydrous titanium perchlorate decomposes explosively at 130 °C and melts at 85 °C with a slight decomposition. It can sublime in a vacuum as low as 70 °C, and can form vapour at up to 120°. Titanium perchlorate is quite volatile. It has density 2.35. It decomposes to TiO2, ClO2 and dioxygen O2 Also TiO(ClO4)2 is formed during decomposition.
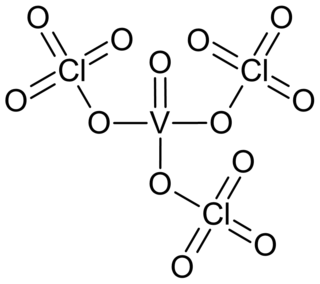
Vanadyl perchlorate or vanadyl triperchlorate is a golden yellow coloured liquid or crystalline compound of vanadium, oxygen and perchlorate group. The substance consists of molecules covalently bound and is quite volatile; it ignites organic solvents on contact and explodes at temperatures above 80 °C.

Rhodium(III) perchlorate refers to the inorganic compound with the formula Rh(H2O)6(ClO4)3. It is a hygroscopic yellow solid. It is the perchlorate salt of the tricationic aquo complex [Rh(H2O)6]3+. The compound is prepared by treating hydrated rhodium(III) chloride and perchloric acid at elevated temperatures:

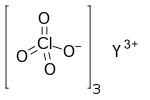
Ytterbium(III) nitrate is an inorganic compound, a salt of ytterbium and nitric acid with the chemical formula Yb(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates.
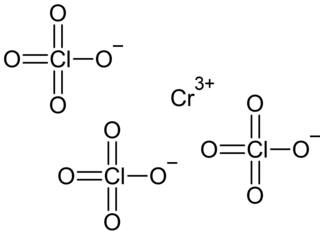
Chromium(III) perchlorate is an inorganic compound with the chemical formula Cr(ClO4)3. It's hexahydrate Cr(ClO4)3·6H2O is a cyan solid that dissolves in water.

Nickel(II) perchlorate is a inorganic compound with the chemical formula of Ni(ClO4)2, and it is a strong oxidizing agent. Its colours are different depending on water. For example, the hydrate forms cyan crystals, the pentahydrate forms green crystals, but the hexahydrate (Ni(ClO4)2·6H2O) forms blue crystals.

Zinc perchlorate is the inorganic compound with the chemical formula Zn(ClO4)2 which forms the hexahydrate.
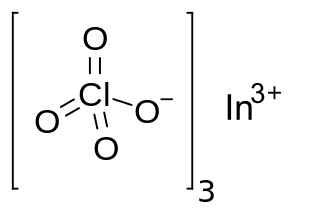
Indium perchlorate is the inorganic compound with the chemical formula In(ClO
4)
3. The compound is an indium salt of perchloric acid.
Terbium perchlorate is an inorganic compound having chemical formula Tb(ClO4)3. This salt of terbium(III) can be obtained by reacting terbium(III,IV) oxide with perchloric acid. The perchlorates are non-coordinating anions, so this substance can be used as a starting material for forming Tb(III) complexes. For example, reaction with alanine forms a complex in which the carboxylate portion of four alanine units bridge between two terbium atoms. It can be used to synthesize terbium-containing metal-organic framework materials.
Samarium(III) perchlorate is an inorganic compound with the chemical formula Sm(ClO4)3.