
Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Europium(III) chloride is an inorganic compound with the formula EuCl3. The anhydrous compound is a yellow solid. Being hygroscopic it rapidly absorbs water to form a white crystalline hexahydrate, EuCl3·6H2O, which is colourless. The compound is used in research.

Diammonium phosphate (DAP; IUPAC name diammonium hydrogen phosphate; chemical formula (NH4)2(HPO4)) is one of a series of water-soluble ammonium phosphate salts that can be produced when ammonia reacts with phosphoric acid.

Ammonium phosphate is the inorganic compound with the formula (NH4)3PO4. It is the ammonium salt of orthophosphoric acid. A related "double salt", (NH4)3PO4.(NH4)2HPO4 is also recognized but is impractical to use. Both triammonium salts evolve ammonia. In contrast to the unstable nature of the triammonium salts, the diammonium phosphate (NH4)2HPO4 and monoammonium salt (NH4)H2PO4 are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus.

Erbium(III) chloride is a violet solid with the formula ErCl3. It is used in the preparation of erbium metal.

Gadolinium(III) chloride, also known as gadolinium trichloride, is GdCl3. It is a colorless, hygroscopic, water-soluble solid. The hexahydrate GdCl3∙6H2O is commonly encountered and is sometimes also called gadolinium trichloride. Gd3+ species are of special interest because the ion has the maximum number of unpaired spins possible, at least for known elements. With seven valence electrons and seven available f-orbitals, all seven electrons are unpaired and symmetrically arranged around the metal. The high magnetism and high symmetry combine to make Gd3+ a useful component in NMR spectroscopy and MRI.

Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters.
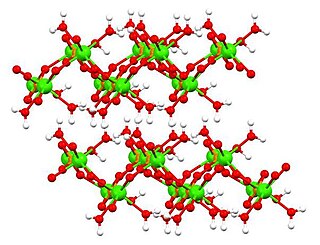
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial.

Zinc bromide (ZnBr2) is an inorganic compound with the chemical formula ZnBr2. It is a colourless salt that shares many properties with zinc chloride (ZnCl2), namely a high solubility in water forming acidic solutions, and good solubility in organic solvents. It is hygroscopic and forms a dihydrate ZnBr2·2H2O.

Yttrium(III) chloride is an inorganic compound of yttrium and chloride. It exists in two forms, the hydrate (YCl3(H2O)6) and an anhydrous form (YCl3). Both are colourless salts that are highly soluble in water and deliquescent.

Lanthanum chloride is the inorganic compound with the formula LaCl3. It is a common salt of lanthanum which is mainly used in research. It is a white solid that is highly soluble in water and alcohols.

Hydrogen phosphate or monohydrogen phosphate(systematic name) is the inorganic ion with the formula [HPO4]2-. Its formula can also be written as [PO3(OH)]2-. Together with dihydrogen phosphate, hydrogenphosphate occurs widely in natural systems. Their salts are used in fertilizers and in cooking. Most hydrogenphosphate salts are colorless, water soluble, and nontoxic.

Thulium(III) chloride or thulium trichloride is as an inorganic salt composed of thulium and chlorine with the formula TmCl3. It forms yellow crystals. Thulium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral thulium ions. It has been used as a starting material for some exotic nanostructures prepared for NIR photocatalysis.

Monofluorophosphate is an anion with the formula PO3F2−, which is a phosphate group with one oxygen atom substituted with a fluoride atom. The charge of the ion is −2. The ion resembles sulfate in size, shape and charge, and can thus form compounds with the same structure as sulfates. These include Tutton's salts and langbeinites. The most well-known compound of monofluorophosphate is sodium monofluorophosphate, commonly used in toothpaste.
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.
Lanthanide trichlorides are a family of inorganic compound with the formula LnCl3, where Ln stands for a lanthanide metal. The trichlorides are standard reagents in applied and academic chemistry of the lanthanides. They exist as anhydrous solids and as hydrates.

Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.

Protactinium(V) fluoride is a fluoride of protactinium with the chemical formula PaF5.



















