
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.
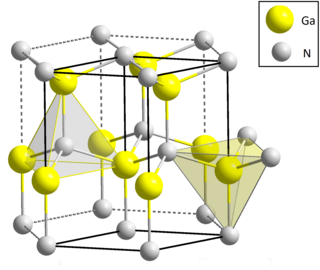
Gallium nitride is a binary III/V direct bandgap semiconductor commonly used in blue light-emitting diodes since the 1990s. The compound is a very hard material that has a Wurtzite crystal structure. Its wide band gap of 3.4 eV affords it special properties for applications in optoelectronic, high-power and high-frequency devices. For example, GaN is the substrate that makes violet (405 nm) laser diodes possible, without requiring nonlinear optical frequency doubling.

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

Aluminium nitride (AlN) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies.
In chemistry, a nitride is an inorganic compound of nitrogen. The "nitride" anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitrides have a found applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common.

Boron trioxide or diboron trioxide is the oxide of boron with the formula B2O3. It is a colorless transparent solid, almost always glassy (amorphous), which can be crystallized only with great difficulty. It is also called boric oxide or boria. It has many important industrial applications, chiefly in ceramics as a flux for glazes and enamels and in the production of glasses.
Indium gallium nitride is a semiconductor material made of a mix of gallium nitride (GaN) and indium nitride (InN). It is a ternary group III/group V direct bandgap semiconductor. Its bandgap can be tuned by varying the amount of indium in the alloy. InxGa1−xN has a direct bandgap span from the infrared for InN to the ultraviolet of GaN. The ratio of In/Ga is usually between 0.02/0.98 and 0.3/0.7.

Silicon nitride is a chemical compound of the elements silicon and nitrogen. Si
3N
4 is the most thermodynamically stable and commercially important of the silicon nitrides, and the term ″Silicon nitride″ commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot H
3PO
4. It is very hard. It has a high thermal stability with strong optical nonlinearities for all-optical applications.

Polyamorphism is the ability of a substance to exist in several different amorphous modifications. It is analogous to the polymorphism of crystalline materials. Many amorphous substances can exist with different amorphous characteristics. However, polyamorphism requires two distinct amorphous states with a clear, discontinuous (first-order) phase transition between them. When such a transition occurs between two stable liquid states, a polyamorphic transition may also be referred to as a liquid–liquid phase transition.

Yttrium arsenide is an inorganic compound of yttrium and arsenic with the chemical formula YAs. It can be prepared by reacting yttrium and arsenic at high temperature. Some literature has done research on the eutectic system of it and zinc arsenide.

Isamu Akasaki was a Japanese engineer and physicist, specializing in the field of semiconductor technology and Nobel Prize laureate, best known for inventing the bright gallium nitride (GaN) p-n junction blue LED in 1989 and subsequently the high-brightness GaN blue LED as well.

Yttrium boride refers to a crystalline material composed of different proportions of yttrium and boron, such as YB2, YB4, YB6, YB12, YB25, YB50 and YB66. They are all gray-colored, hard solids having high melting temperatures. The most common form is the yttrium hexaboride YB6. It exhibits superconductivity at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating for low-energy synchrotron radiation (1–2 keV).

The 122 iron arsenide unconventional superconductors are part of a new class of iron-based superconductors. They form in the tetragonal I4/mmm, ThCr2Si2 type, crystal structure. The shorthand name "122" comes from their stoichiometry; the 122s have the chemical formula AEFe2Pn2, where AE stands for alkaline earth metal (Ca, Ba Sr or Eu) and Pn is pnictide (As, P, etc.). These materials become superconducting under pressure and also upon doping. The maximum superconducting transition temperature found to date is 38 K in the Ba0.6K0.4Fe2As2. The microscopic description of superconductivity in the 122s is yet unclear.

Germanene is a material made up of a single layer of germanium atoms. The material is created in a process similar to that of silicene and graphene, in which high vacuum and high temperature are used to deposit a layer of germanium atoms on a substrate. High-quality thin films of germanene have revealed unusual two-dimensional structures with novel electronic properties suitable for semiconductor device applications and materials science research.
Oxyarsenides or arsenide oxides are chemical compounds formally containing the group AsO, with one arsenic and one oxygen atom. The arsenic and oxygen are not bound together as in arsenates or arsenites, instead they make a separate presence bound to the cations (metals), and could be considered as a mixed arsenide-oxide compound. So a compound with OmAsn requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxypnictide compounds.
Gallium(III) sulfate refers to the chemical compound, a salt, with the formula Ga2(SO4)3, or its hydrates Ga2(SO4)3·xH2O. Gallium metal dissolves in sulfuric acid to form solutions containing [Ga(OH2)6]3+ and SO42− ions. The octadecahydrate Ga2(SO4)3·18H2O crystallises from these solutions at room temperature. This hydrate loses water in stages when heated, forming the anhydrate Ga2(SO4)3 above 150 °C and completely above 310 °C. Anhydrous Ga2(SO4)3 is isostructural with iron(III) sulfate, crystallizing in the rhombohedral space group R3.
Silicide carbides or carbide silicides are compounds containing anions composed of silicide (Si4−) and carbide (C4−) or clusters therof. They can be considered as mixed anion compounds or intermetallic compounds, as silicon could be considered as a semimetal.
Protactinium nitride is a binary inorganic compound of protactinium and nitrogen with the chemical formula PaN.
















