Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colourless and odourless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.
In chemistry, a nitride is a chemical compound of nitrogen. Nitrides can be inorganic or organic, ionic or covalent. The nitride anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitrides have a found applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common.
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called comproportionation, also known as symproportionation.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
In inorganic chemistry, chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond. Trichlorosilane is produced on the largest scale. The parent chlorosilane is silicon tetrachloride.

Phosphorus sulfides comprise a family of inorganic compounds containing only phosphorus and sulfur. These compounds have the formula P4Sn with n ≤ 10. Two are of commercial significance, phosphorus pentasulfide, which is made on a kiloton scale for the production of other organosulfur compounds, and phosphorus sesquisulfide, used in the production of "strike anywhere matches".

Sulfur dichloride is the chemical compound with the formula SCl2. This cherry-red liquid is the simplest sulfur chloride and one of the most common, and it is used as a precursor to organosulfur compounds. It is a highly corrosive and toxic substance, and it reacts on contact with water to form chlorine-containing acids.
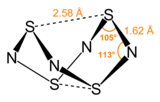
Sulfur nitride may refer to a number of sulfur nitrogen compounds:

Sulfur tetrafluoride is a chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Disulfur dichloride is the inorganic compound of sulfur and chlorine with the formula S2Cl2. It is an amber oily liquid.
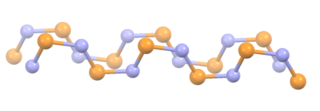
Polythiazyl, (SN)x, is an electrically conductive, gold- or bronze-colored polymer with metallic luster. It was the first conductive inorganic polymer discovered and was also found to be a superconductor at very low temperatures. It is a fibrous solid, described as "lustrous golden on the faces and dark blue-black", depending on the orientation of the sample. It is air stable and insoluble in all solvents.
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.
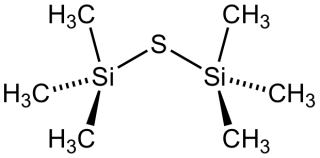
Bis(trimethylsilyl) sulfide is the chemical compound with the formula ((CH3)3Si)2S. Often abbreviated (tms)2S, this colourless, vile-smelling liquid is a useful aprotic source of "S2−" in chemical synthesis.

Bis(triphenylphosphine)iminium chloride is the chemical compound with the formula [( 3P)2N]Cl, often abbreviated [(Ph3P)2N]Cl, where Ph is phenyl C6H5, or even abbreviated [PPN]Cl or [PNP]Cl or PPNCl or PNPCl, where PPN or PNP stands for (Ph3P)2N. This colorless salt is a source of the [(Ph3P)2N]+ cation, which is used as an unreactive and weakly coordinating cation to isolate reactive anions. [(Ph3P)2N]+ is a phosphazene.

Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(Si(CH3)3)2. It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.

Thiazyl fluoride, NSF, is a colourless, pungent gas at room temperature and condenses to a pale yellow liquid at 0.4 °C. Along with thiazyl trifluoride, NSF3, it is an important precursor to sulfur-nitrogen-fluorine compounds. It is notable for its extreme hygroscopicity.

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal M with anionic bis(trimethylsilyl)amide ligands (the −N 2 monovalent anion, or −N 2 monovalent group, and are part of a broader category of metal amides.

Tris(trimethylsilyl)amine is the simplest tris(trialkylsilyl)amine which are having the general formula (R3Si)3N, in which all three hydrogen atoms of the ammonia are replaced by trimethylsilyl groups (-Si(CH3)3). Tris(trimethylsilyl)amine has been for years in the center of scientific interest as a stable intermediate in chemical nitrogen fixation (i. e. the conversion of atmospheric nitrogen N2 into organic substrates under normal conditions).

Trithiazyl trichloride is the inorganic compound with the formula (NSCl)3. A white solid, it is a precursor to other sulfur nitrides, but has no commercial applications.
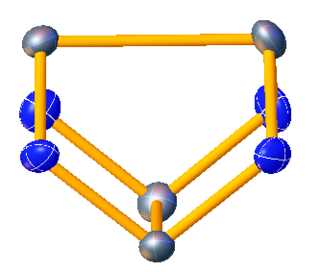
Tetraselenium tetranitride is the inorganic compound with the formula Se4N4. Like the analogous tetrasulfur tetranitride S4N4, Se4N4 is an orange solid. It is however less soluble and more shock-sensitive than S4N4.