Bioleaching is the extraction or liberation of metals from their ores through the use of living organisms. Bioleaching is one of several applications within biohydrometallurgy and several methods are used to treat ores or concentrates containing copper, zinc, lead, arsenic, antimony, nickel, molybdenum, gold, silver, and cobalt.

Smelting is a process of applying heat and a chemical reducing agent to an ore to extract a desired base metal product. It is a form of extractive metallurgy that is used to obtain many metals such as iron, copper, silver, tin, lead and zinc. Smelting uses heat and a chemical reducing agent to decompose the ore, driving off other elements as gases or slag and leaving the metal behind. The reducing agent is commonly a fossil-fuel source of carbon, such as carbon monoxide from incomplete combustion of coke—or, in earlier times, of charcoal. The oxygen in the ore binds to carbon at high temperatures, as the chemical potential energy of the bonds in carbon dioxide is lower than that of the bonds in the ore.
Extractive metallurgy is a branch of metallurgical engineering wherein process and methods of extraction of metals from their natural mineral deposits are studied. The field is a materials science, covering all aspects of the types of ore, washing, concentration, separation, chemical processes and extraction of pure metal and their alloying to suit various applications, sometimes for direct use as a finished product, but more often in a form that requires further working to achieve the given properties to suit the applications.

Pentlandite is an iron–nickel sulfide with the chemical formula (Fe,Ni)9S8. Pentlandite has a narrow variation range in nickel to iron ratios (Ni:Fe), but it is usually described as 1:1. In some cases, this ratio is skewed by the presence of pyrrhotite inclusions. It also contains minor cobalt, usually at low levels as a fraction of weight.

Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.

Nickeline or niccolite is the mineral form of nickel arsenide. The naturally-occurring mineral contains roughly 43.9% nickel and 56.1% arsenic by mass, but composition of the mineral may vary slightly.

Copper extraction refers to the methods used to obtain copper from its ores. The conversion of copper ores consists of a series of physical, chemical, and electrochemical processes. Methods have evolved and vary with country depending on the ore source, local environmental regulations, and other factors.

Chalcocite, copper(I) sulfide (Cu2S), is an important copper ore mineral. It is opaque and dark gray to black, with a metallic luster. It has a hardness of 2.5–3 on the Mohs scale. It is a sulfide with a monoclinic crystal system.

Cobaltite is an arsenide and sulfide mineral with the mineral formula CoAsS. It is the naming mineral of the cobaltite group of minerals, whose members structurally resemble pyrite (FeS2).

Fukuchilite, Cu
3FeS
8, is a copper iron sulfide named after the Japanese mineralogist Nobuyo Fukuchi (1877–1934), that occurs in ore bodies of gypsum-anhydrite at the intersection points of small masses of barite, covellite, gypsum and pyrite, and is mostly found in the Hanawa mine in the Akita prefecture of Honshū, Japan where it was first discovered in 1969. It occurs in masses within the third geologic unit of the Kuroko type deposits within the mine.
In ore deposit geology, supergene processes or enrichment are those that occur relatively near the surface as opposed to deep hypogene processes. Supergene processes include the predominance of meteoric water circulation (i.e. water derived from precipitation) with concomitant oxidation and chemical weathering. The descending meteoric waters oxidize the primary (hypogene) sulfide ore minerals and redistribute the metallic ore elements. Supergene enrichment occurs at the base of the oxidized portion of an ore deposit. Metals that have been leached from the oxidized ore are carried downward by percolating groundwater, and react with hypogene sulfides at the supergene-hypogene boundary. The reaction produces secondary sulfides with metal contents higher than those of the primary ore. This is particularly noted in copper ore deposits where the copper sulfide minerals chalcocite (Cu2S), covellite (CuS), digenite (Cu18S10), and djurleite (Cu31S16) are deposited by the descending surface waters.
Biomining refers to any process that uses living organisms to extract metals from ores and other solid materials. Typically these processes involve prokaryotes, however fungi and plants may also be used. Biomining is one of several applications within biohydrometallurgy with applications in ore refinement, precious metal recovery, and bioremediation. The largest application currently being used is the treatment of mining waste containing iron, copper, zinc, and gold allowing for salvation of any discarded minerals. It may also be useful in maximizing the yields of increasingly low grade ore deposits. Biomining has been proposed as a relatively environmentally friendly alternative and/or supplementation to traditional mining. Current methods of biomining are modified leach mining processes. These aptly named bioleaching processes most commonly includes the inoculation of extracted rock with bacteria and acidic solution, with the leachate salvaged and processed for the metals of value. Biomining has many applications outside of metal recovery, most notably is bioremediation which has already been used to clean up coastlines after oil spills. There are also many promising future applications, like space biomining, fungal bioleaching and biomining with hybrid biomaterials.
Mount Isa Mines Limited ("MIM") operates the Mount Isa copper, lead, zinc and silver mines near Mount Isa, Queensland, Australia as part of the Glencore group of companies. For a brief period in 1980, MIM was Australia's largest company. It has pioneered several significant mining industry innovations, including the Isa Process copper refining technology, the Isasmelt smelting technology, and the IsaMill fine grinding technology, and it also commercialized the Jameson Cell column flotation technology.
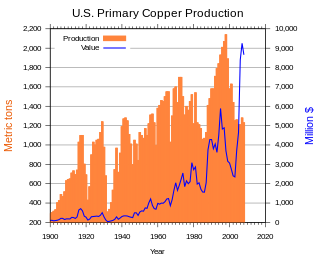
In the United States, copper mining has been a major industry since the rise of the northern Michigan copper district in the 1840s. In 2017, the US produced 1.27 million metric tonnes of copper, worth $8 billion, making it the world's fourth largest copper producer, after Chile, China, and Peru. Copper was produced from 23 mines in the US. Top copper producing states in 2014 were Arizona, Utah, New Mexico, Nevada, and Montana. Minor production also came from Idaho and Missouri. As of 2014, the US had 45 million tonnes of known remaining reserves of copper, the fifth largest known copper reserves in the world, after Chile, Australia, Peru, and Mexico.
Leaching is a process widely used in extractive metallurgy where ore is treated with chemicals to convert the valuable metals within the ore, into soluble salts while the impurity remains insoluble. These can then be washed out and processed to give the pure metal; the materials left over are commonly known as tailings.

Cubanite is a copper iron sulfide mineral that commonly occurs as a minor alteration mineral in magmatic sulfide deposits. It has the chemical formula CuFe2S3 and when found, it has a bronze to brass-yellow appearance. On the Mohs hardness scale, cubanite falls between 3.5 and 4 and has a orthorhombic crystal system. Cubanite is chemically similar to chalcopyrite; however, it is the less common copper iron sulfide mineral due to crystallization requirements.

Cobalt extraction refers to the techniques used to extract cobalt from its ores and other compound ores. Several methods exist for the separation of cobalt from copper and nickel. They depend on the concentration of cobalt and the exact composition of the ore used.

Mopani Copper Mines PLC is a Zambian company that produces and sells copper and cobalt to the international market, being one of the biggest mines and exporters in the world.

The Manhès–David process is a refining process of the copper mattes, invented in 1880 by the French industrialist Pierre Manhès and his engineer Paul David. Inspired by the Bessemer process, it consists of the use of a converter to oxidise with air the undesirable chemical elements contained in the matte, to transform it into copper.
Mineral processing and extraction of metals are very energy-intensive processes, which are not exempted of producing large volumes of solid residues and wastewater, which also require energy to be further treated and disposed. Moreover, as the demand for metals increases, the metallurgical industry must rely on sources of materials with lower metal contents both from a primary and/or secondary raw materials. Consequently, mining activities and waste recycling must evolve towards the development of more selective, efficient and environmentally friendly mineral and metal processing routes.


















