Smelting is a process of applying heat and a chemical reducing agent to an ore to extract a desired base metal product. It is a form of extractive metallurgy that is used to obtain many metals such as iron, copper, silver, tin, lead and zinc. Smelting uses heat and a chemical reducing agent to decompose the ore, driving off other elements as gases or slag and leaving the metal behind. The reducing agent is commonly a fossil-fuel source of carbon, such as carbon monoxide from incomplete combustion of coke—or, in earlier times, of charcoal. The oxygen in the ore binds to carbon at high temperatures, as the chemical potential energy of the bonds in carbon dioxide is lower than that of the bonds in the ore.

Chalcopyrite ( KAL-kə-PY-ryte, -koh-) is a copper iron sulfide mineral and the most abundant copper ore mineral. It has the chemical formula CuFeS2 and crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green-tinged black.

The general term slag may be a by-product or co-product of smelting (pyrometallurgical) ores and recycled metals depending on the type of material being produced. Slag is mainly a mixture of metal oxides and silicon dioxide. Broadly, it can be classified as ferrous, ferroalloy or non-ferrous/base metals. Within these general categories, slags can be further categorized by their precursor and processing conditions. Slag generated from the EAF process can contain toxic metals, which can be hazardous to human and environmental health.
A metallurgical method employed in the purification of copper which contains copper oxide as an impurity and also in the purification of tin which contains tin oxide (stannic oxide or "SnO2") as an impurity. The impure metal, usually in the form of molten blister copper, is placed in an anode furnace for two stages of refining. In the first stage, sulphur and iron are removed by gently blowing air through the molten metal to form iron oxides and sulfur dioxide. The iron oxides are skimmed or poured off the top of the copper and the gaseous sulfur dioxide exits the furnace via the off-gas system. Once the first oxidation stage is complete, the second stage (reduction or poling) begins. This involves using a reducing agent, normally natural gas or diesel (but ammonia, liquid petroleum gas, and naphtha can also be used), to react with the oxygen in the copper oxide to form copper. In the past, freshly cut ("green") trees were used as wooden poles. The sap in these poles acted as the reducing agent. The heat of the copper makes the pole emit wood gas(CO2 and H2) that reduces the cuprous oxide to copper.

Industrial processes are procedures involving chemical, physical, electrical, or mechanical steps to aid in the manufacturing of an item or items, usually carried out on a very large scale. Industrial processes are the key components of heavy industry.

Copper extraction refers to the methods used to obtain copper from its ores. The conversion of copper ores consists of a series of physical, chemical and electrochemical processes. Methods have evolved and vary with country depending on the ore source, local environmental regulations, and other factors.

Basic oxygen steelmaking, also known as Linz-Donawitz steelmaking or the oxygen converter process, is a method of primary steelmaking in which carbon-rich molten pig iron is made into steel. Blowing oxygen through molten pig iron lowers the carbon content of the alloy and changes it into low-carbon steel. The process is known as basic because fluxes of calcium oxide or dolomite, which are chemical bases, are added to promote the removal of impurities and protect the lining of the converter.
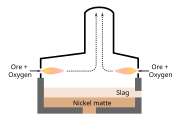
Pyrometallurgy is a branch of extractive metallurgy. It consists of the thermal treatment of minerals and metallurgical ores and concentrates to bring about physical and chemical transformations in the materials to enable recovery of valuable metals. Pyrometallurgical treatment may produce products able to be sold such as pure metals, or intermediate compounds or alloys, suitable as feed for further processing. Examples of elements extracted by pyrometallurgical processes include the oxides of less reactive elements like iron, copper, zinc, chromium, tin, and manganese.

Kennecott Utah Copper LLC’s Garfield Smelter Stack is a 1,215-foot (370 m) high smokestack west of Magna, Utah, alongside Interstate 80 near the Great Salt Lake. It was built to disperse exhaust gases from the Kennecott Utah Copper smelter at Garfield, Utah. It is the 60th-tallest freestanding structure in the world, the 4th-tallest chimney, and the tallest man-made structure west of the Mississippi River.

Roasting is a process of heating a sulfide ore to a high temperature in the presence of air. It is a step in the processing of certain ores. More specifically, roasting is often a metallurgical process involving gas–solid reactions at elevated temperatures with the goal of purifying the metal component(s). Often before roasting, the ore has already been partially purified, e.g. by froth flotation. The concentrate is mixed with other materials to facilitate the process. The technology is useful in making certain ores usable but it can also be a serious source of air pollution.
In metallurgy, refining consists of purifying an impure metal. It is to be distinguished from other processes such as smelting and calcining in that those two involve a chemical change to the raw material, whereas in refining the final material is chemically identical to the raw material. Refining thus increases the purity of the raw material via processing. There are many processes including pyrometallurgical and hydrometallurgical techniques.
Mount Isa Mines Limited ("MIM") operates the Mount Isa copper, lead, zinc and silver mines near Mount Isa, Queensland, Australia as part of the Glencore group of companies. For a brief period in 1980, MIM was Australia's largest company. It has pioneered several significant mining industry innovations, including the Isa Process copper refining technology, the Isasmelt smelting technology, and the IsaMill fine grinding technology, and it also commercialized the Jameson Cell column flotation technology.
Zinc smelting is the process of converting zinc concentrates into pure zinc. Zinc smelting has historically been more difficult than the smelting of other metals, e.g. iron, because in contrast, zinc has a low boiling point. At temperatures typically used for smelting metals, zinc is a gas that will escape from a furnace with the flue gas and be lost, unless specific measures are taken to prevent it.

Archaeometallurgical slag is slag discovered and studied in the context of archaeology. Slag, the byproduct of iron-working processes such as smelting or smithing, is left at the iron-working site rather than being moved away with the product. As it weathers well, it is readily available for study. The size, shape, chemical composition and microstructure of slag are determined by features of the iron-working processes used at the time of its formation.

Cobalt extraction refers to the techniques used to extract cobalt from its ores and other compound ores. Several methods exist for the separation of cobalt from copper and nickel. They depend on the concentration of cobalt and the exact composition of the ore used.

Plants for the production of lead are generally referred to as lead smelters. Primary lead production begins with sintering. Concentrated lead ore is fed into a sintering machine with iron, silica, limestone fluxes, coke, soda ash, pyrite, zinc, caustics or pollution control particulates. Smelting uses suitable reducing substances that will combine with those oxidizing elements to free the metal. Reduction is the final, high-temperature step in smelting. It is here that the oxide becomes the elemental metal. A reducing environment pulls the final oxygen atoms from the raw metal.

The ISASMELT process is an energy-efficient smelting process that was jointly developed from the 1970s to the 1990s by Mount Isa Mines and the Government of Australia's CSIRO. It has relatively low capital and operating costs for a smelting process.

The Bottom-blown Oxygen Converter or BBOC is a smelting furnace developed by the staff at Britannia Refined Metals Limited (“BRM”), a British subsidiary of MIM Holdings Limited. The furnace is currently marketed by Glencore Technology. It is a sealed, flat-bottomed furnace mounted on a tilting frame that is used in the recovery of precious metals. A key feature is the use of a shrouded lance to inject oxygen through the bottom of the furnace, directly into the precious metals contained in the furnace, to oxidize base metals or other impurities as part of their removal as slag.
A Kaldo converter is a rotary vessel oxygen based metal refining method. Originally applied to the refining of iron into steel, with most installations in the 1960s, the process is (2014) used primarily to refine non ferrous metals, typically copper. In that field, it is often named TBRC, or Top Blown Rotary Converter.

The Manhès–David process is a refining process of the copper mattes, invented in 1880 by the French industrialist Pierre Manhès and his engineer Paul David. Inspired by the Bessemer process, it consists of the use of a converter to oxidise with air the undesirable chemical elements contained in the matte, to transform it into copper.