
Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in 1822 by Leopold Gmelin.
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds—that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as inorganic chemistry.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

Sodium amide, commonly called sodamide, is the inorganic compound with the formula NaNH2. It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent. NaNH2 conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. NaNH2 has been widely employed as a strong base in organic synthesis.

Sodium amalgam, with the common formula Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions involving mercury as a major component. Sodium amalgams are often used in reactions as strong reducing agents with better handling properties compared to solid sodium. They are less dangerously reactive toward water and in fact are often used as an aqueous suspension.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.
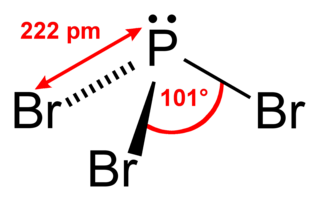
Phosphorus tribromide is a colourless liquid with the formula PBr3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the laboratory for the conversion of alcohols to alkyl bromides.
Phosphorus trifluoride (formula PF3), is a colorless and odorless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls, and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide.

Sulfur dichloride is the chemical compound with the formula SCl2. This cherry-red liquid is the simplest sulfur chloride and one of the most common, and it is used as a precursor to organosulfur compounds. It is a highly corrosive and toxic substance, and it reacts on contact with water to form chlorine-containing acids.

Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Sulfur tetrafluoride is a chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Vanadium(II) chloride is the inorganic compound with the formula VCl2, and is the most reduced vanadium chloride. Vanadium(II) chloride is an apple-green solid that dissolves in water to give purple solutions.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.

Lead(IV) acetate or lead tetraacetate is an metalorganic compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride.
Sodium hypobromite is an inorganic compound with the chemical formula NaOBr. It is a sodium salt of hypobromous acid. It consists of sodium cations Na+ and hypobromite anions −OBr. It is usually obtained as the pentahydrate, so the compound that is usually called sodium hypobromite actually has the formula NaBrO·5H2O. It is a yellow-orange solid that is soluble in water. It adopts a monoclinic crystal structure with a Br–O bond length of 1.820 Å. It is the bromine analogue of sodium hypochlorite, the active ingredient in common bleach. In practice the salt is usually encountered as an aqueous solution.

Azanide is the IUPAC-sanctioned name for the anion NH−2. The term is obscure; derivatives of NH−2 are almost invariably referred to as amides, despite the fact that amide also refers to the organic functional group –C(=O)−NR2. The anion NH−2 is the conjugate base of ammonia, so it is formed by the self-ionization of ammonia. It is produced by deprotonation of ammonia, usually with strong bases or an alkali metal. Azanide has a H–N–H bond angle of 104.5°.

Lithium naphthalene is an organic salt with the chemical formula Li+[C10H8]−. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. Lithium naphthalene crystallizes with ligands bound to Li+. The anion is a well-known example of an organic radical.















