
Europium is a chemical element; it has symbol Eu and atomic number 63. Europium is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. It is the most chemically reactive, least dense, and softest of the lanthanide elements. It is soft enough to be cut with a knife. Europium was isolated in 1901 and named after the continent of Europe. Europium usually assumes the oxidation state +3, like other members of the lanthanide series, but compounds having oxidation state +2 are also common. All europium compounds with oxidation state +2 are slightly reducing. Europium has no significant biological role and is relatively non-toxic compared to other heavy metals. Most applications of europium exploit the phosphorescence of europium compounds. Europium is one of the rarest of the rare-earth elements on Earth.
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (HS−) are the conjugate acids of sulfide.

Zinc sulfide is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics.

Lead(II) sulfide is an inorganic compound with the formula PbS. Galena is the principal ore and the most important compound of lead. It is a semiconducting material with niche uses.

Europium(III) chloride is an inorganic compound with the formula EuCl3. The anhydrous compound is a yellow solid. Being hygroscopic it rapidly absorbs water to form a white crystalline hexahydrate, EuCl3·6H2O, which is colourless. The compound is used in research.

Sodium hydrosulfide is the chemical compound with the formula NaSH. This compound is the product of the half-neutralization of hydrogen sulfide with sodium hydroxide (NaOH). NaSH and sodium sulfide are used industrially, often for similar purposes. Solid NaSH is colorless. The solid has an odor of H2S owing to hydrolysis by atmospheric moisture. In contrast with sodium sulfide, which is insoluble in organic solvents, NaSH, being a 1:1 electrolyte, is more soluble.

Ruthenium(IV) oxide is the inorganic compound with the formula RuO2. This black solid is the most common oxide of ruthenium. It is widely used as an electrocatalyst for producing chlorine, chlorine oxides, and O2. Like many dioxides, RuO2 adopts the rutile structure.
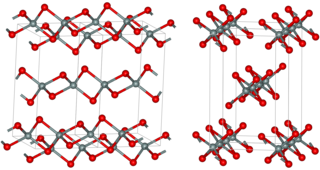
Silicon disulfide is the inorganic compound with the formula SiS2. Like silicon dioxide, this material is polymeric, but it adopts a 1-dimensional structure quite different from the usual forms of SiO2.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.
Thiosulfuric acid is the inorganic compound with the formula H2S2O3. It has attracted academic interest as a simple, easily accessed compound that is labile. It has few practical uses.

Copper(I) sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral chalcocite. It has a narrow range of stoichiometry ranging from Cu1.997S to Cu2.000S. Samples are typically black.

Titanium disulfide is an inorganic compound with the formula TiS2. A golden yellow solid with high electrical conductivity, it belongs to a group of compounds called transition metal dichalcogenides, which consist of the stoichiometry ME2. TiS2 has been employed as a cathode material in rechargeable batteries.

Nickel sulfide is any inorganic compound with the formula NixSy. These compounds range in color from bronze (Ni3S2) to black (NiS2). The nickel sulfide with simplest stoichiometry is NiS, also known as the mineral millerite. From the economic perspective, Ni9S8, the mineral pentlandite, is the chief source of mined nickel. Other minerals include heazlewoodite (Ni3S2) and polydymite (Ni3S4), and the mineral Vaesite (NiS2). Some nickel sulfides are used commercially as catalysts.

Europium(III) nitrate is an inorganic compound with the formula Eu(NO3)3·x(H2O). The hexahydrate is a common salt. It forms colorless hygroscopic crystals.
Sulfidostannates, or thiostannates are chemical compounds containing anions composed of tin linked with sulfur. They can be considered as stannates with sulfur substituting for oxygen. Related compounds include the thiosilicates, and thiogermanates, and by varying the chalcogen: selenostannates, and tellurostannates. Oxothiostannates have oxygen in addition to sulfur. Thiostannates can be classed as chalcogenidometalates, thiometallates, chalcogenidotetrelates, thiotetrelates, and chalcogenidostannates. Tin is almost always in the +4 oxidation state in thiostannates, although a couple of mixed sulfides in the +2 state are known,

Europium(II) oxide (EuO) is a chemical compound which is one of the oxides of europium. In addition to europium(II) oxide, there is also europium(III) oxide and the mixed valence europium(II,III) oxide.

Europium(III) acetate is an inorganic salt of europium and acetic acid with the chemical formula of Eu(CH3COO)3. In this compound, europium exhibits the +3 oxidation state. It can exist in the anhydrous form, sesquihydrate and tetrahydrate. Its hydrate molecule is a dimer.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.
Lanthanide compounds are compounds formed by the 15 elements classed as lanthanides. The lanthanides are generally trivalent, although some, such as cerium and europium, are capable of forming compounds in other oxidation states.
















