Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.
Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.

Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as halides of carbon without carbon-hydrogen and carbon-carbon bonds, and certain compounds of carbon with nitrogen and oxygen.
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds—that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as inorganic chemistry.

A clathrate is a chemical substance consisting of a lattice that traps or contains molecules. The word clathrate is derived from the Latin clathratus, meaning 'with bars, latticed'. Most clathrate compounds are polymeric and completely envelop the guest molecule, but in modern usage clathrates also include host–guest complexes and inclusion compounds. According to IUPAC, clathrates are inclusion compounds "in which the guest molecule is in a cage formed by the host molecule or by a lattice of host molecules." The term refers to many molecular hosts, including calixarenes and cyclodextrins and even some inorganic polymers such as zeolites.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.
Lithotrophs are a diverse group of organisms using an inorganic substrate to obtain reducing equivalents for use in biosynthesis or energy conservation via aerobic or anaerobic respiration. While lithotrophs in the broader sense include photolithotrophs like plants, chemolithotrophs are exclusively microorganisms; no known macrofauna possesses the ability to use inorganic compounds as electron sources. Macrofauna and lithotrophs can form symbiotic relationships, in which case the lithotrophs are called "prokaryotic symbionts". An example of this is chemolithotrophic bacteria in giant tube worms or plastids, which are organelles within plant cells that may have evolved from photolithotrophic cyanobacteria-like organisms. Chemolithotrophs belong to the domains Bacteria and Archaea. The term "lithotroph" was created from the Greek terms 'lithos' (rock) and 'troph' (consumer), meaning "eaters of rock". Many but not all lithoautotrophs are extremophiles.
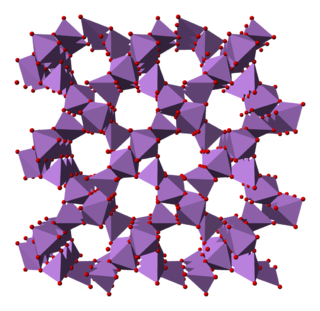
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
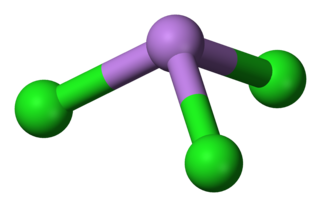
Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.

A lithoautotroph is an organism which derives energy from reactions of reduced compounds of mineral (inorganic) origin. Two types of lithoautotrophs are distinguished by their energy source; photolithoautotrophs derive their energy from light while chemolithoautotrophs (chemolithotrophs or chemoautotrophs) derive their energy from chemical reactions. Chemolithoautotrophs are exclusively microbes. Photolithoautotrophs include macroflora such as plants; these do not possess the ability to use mineral sources of reduced compounds for energy. Most chemolithoautotrophs belong to the domain Bacteria, while some belong to the domain Archaea. Lithoautotrophic bacteria can only use inorganic molecules as substrates in their energy-releasing reactions. The term "lithotroph" is from Greek lithos (λίθος) meaning "rock" and trōphos (τροφοσ) meaning "consumer"; literally, it may be read "eaters of rock". The "lithotroph" part of the name refers to the fact that these organisms use inorganic elements/compounds as their electron source, while the "autotroph" part of the name refers to their carbon source being CO2. Many lithoautotrophs are extremophiles, but this is not universally so, and some can be found to be the cause of acid mine drainage.
The Gmelin database is a large database of organometallic and inorganic compounds updated quarterly. It is based on the German publication Gmelins Handbuch der anorganischen Chemie which was originally published by Leopold Gmelin in 1817; the last print edition, the 8th, appeared in the 1990s. Although published over many decades, the printed series was not uniform in coverage or currency. Some elements are represented only by decades-old and not updated slim summary volumes. Others have numerous supplements. Most later supplement volumes focused on an element's organic complexes. Each volume lists its literature coverage date.
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among all possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations.

An autotroph is an organism that can convert abiotic sources of energy into energy stored in organic compounds, which can be used by other organisms. Autotrophs produce complex organic compounds using carbon from simple substances such as carbon dioxide, generally using energy from light or inorganic chemical reactions. Autotrophs do not need a living source of carbon or energy and are the producers in a food chain, such as plants on land or algae in water. Autotrophs can reduce carbon dioxide to make organic compounds for biosynthesis and as stored chemical fuel. Most autotrophs use water as the reducing agent, but some can use other hydrogen compounds such as hydrogen sulfide.

Norman Neill Greenwood FRS CChem FRSC was an Australian-British chemist and Emeritus Professor at the University of Leeds. Together with Alan Earnshaw, he wrote the textbook Chemistry of the Elements, first published in 1984.
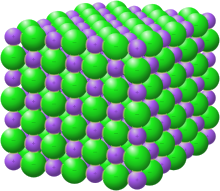
Sodium bifluoride is the inorganic compound with the formula Na[HF2]. It is a salt of sodium cation and bifluoride anion. It is a white, water-soluble solid that decomposes upon heating. Sodium bifluoride is non-flammable, hygroscopic, and has a pungent smell. Sodium bifluoride has a number of applications in industry.

Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.

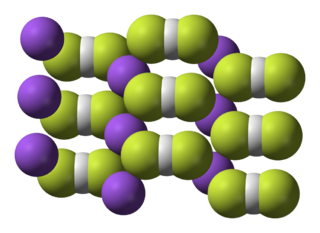
Xenon tetrachloride is an unstable inorganic compound with the chemical formula XeCl4. Unlike other noble gas/halide compounds, it cannot be synthesized by simply combining the elements, by using a more-active halogenating agent, or by substitution of other halides on tetrahaloxenon compounds. Instead, a decay technique can be used, starting with K129ICl4. The iodine-129 atom of the 129
ICl–
4 covalent cluster is radioactive and undergoes beta decay to become xenon-129. The resulting XeCl4 molecule has a square planar molecular geometry analogous to xenon tetrafluoride.
Polonium tetraiodide is a binary inorganic compound of polonium and iodine with the chemical formula PoI
4. The compound forms volatile black crystals.