
In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

Copper monosulfide is a chemical compound of copper and sulfur. It was initially thought to occur in nature as the dark indigo blue mineral covellite. However, it was later shown to be rather a cuprous compound, formula Cu+3S(S2). CuS is a moderate conductor of electricity. A black colloidal precipitate of CuS is formed when hydrogen sulfide, H2S, is bubbled through solutions of Cu(II) salts. It is one of a number of binary compounds of copper and sulfur (see copper sulfide for an overview of this subject), and has attracted interest because of its potential uses in catalysis and photovoltaics.

Vanadium(III) chloride is the inorganic compound with the formula VCl3 which forms the hexahydrate, [VCl2(H2O)4]Cl·2H2O. This hygroscopic purple salt is a common precursor to other vanadium(III) complexes.

Mercury(II) thiocyanate (Hg(SCN)2) is an inorganic chemical compound, the coordination complex of Hg2+ and the thiocyanate anion. It is a white powder. It will produce a large, winding "snake" when ignited, an effect known as the Pharaoh's serpent.
Indium(III) sulfide (Indium sesquisulfide, Indium sulfide (2:3), Indium (3+) sulfide) is the inorganic compound with the formula In2S3.
Samarium(III) sulfide (Sm2S3) is a chemical compound of the rare earth element samarium, and sulfur. In this compound samarium is in the +3 oxidation state, and sulfur is an anion in the −2 state.
Antimony pentasulfide is an inorganic compound of antimony and sulfur, also known as antimony red. It is a nonstoichiometric compound with a variable composition. Its structure is unknown. Commercial samples are contaminated with sulfur, which may be removed by washing with carbon disulfide in a Soxhlet extractor.
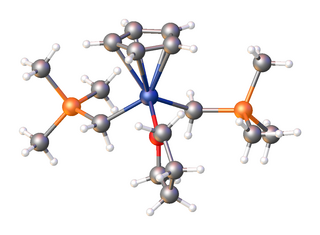
Organoscandium chemistry is an area with organometallic compounds focused on compounds with at least one carbon to scandium chemical bond. The interest in organoscandium compounds is mostly academic but motivated by potential practical applications in catalysis, especially in polymerization. A common precursor is scandium chloride, especially its THF complex.

Potassium hydrosulfide is the inorganic compound with the formula KSH. This colourless salt consists of the cation K+ and the bisulfide anion [SH]−. It is the product of the half-neutralization of hydrogen sulfide with potassium hydroxide. The compound is used in the synthesis of some organosulfur compounds. Aqueous solutions of potassium sulfide consist of a mixture of potassium hydrosulfide and potassium hydroxide.

Scandium monosulfide is a chemical compound of scandium and sulfur with the chemical formula ScS. Although its formula might suggest that it is a compound of scandium(II), i.e. [Sc2+][S2−], ScS is probably more realistically described as a pseudo-ionic compound, containing [Sc3+][S2−], with the remaining electron occupying the conduction band of the solid.

tert-Butylthiol, also known as 2-methylpropane-2-thiol, 2-methyl-2-propanethiol, tert-butyl mercaptan (TBM), and t-BuSH, is an organosulfur compound with the formula (CH3)3CSH. This thiol is used as an odorant for natural gas, which is otherwise odorless. It may also have been used as a flavoring agent.

Berkelium forms a number of chemical compounds, where it normally exists in an oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid. Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halogens, chalcogens and pnictogens to form various binary compounds. Berkelium can also form several organometallic compounds.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.
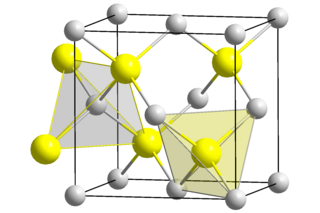
Beryllium sulfide (BeS) is an ionic compound from the sulfide group with the formula BeS. It is a white solid with a sphalerite structure that is decomposed by water and acids.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.

Rhodium(III) sulfide is the inorganic compound with the formula Rh2S3. It is an insoluble black solid, prepared by the heating a mixture of elemental rhodium and sulfur. Crystals can be grown by chemical vapor transport using bromine as the transporting agent. The structure consists of octahedral and tetrahedral Rh and S centers, respectively. No close Rh-Rh contacts are observed. Rh2Se3 and Ir2S3 adopt the same structure as Rh2S3.
Mixed-anion compounds, heteroanionic materials or mixed-anion materials are chemical compounds containing cations and more than one kind of anion. The compounds contain a single phase, rather than just a mixture.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +6, +4, and +2 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. Tetrathioperrhenate anion [ReS4]− is possible.
Protactinium compounds are compounds containing the element protactinium. These compounds usually have protactinium in the +5 oxidation state, although these compounds can also exist in the +2, +3 and +4 oxidation states.














