
Iridium is a chemical element; it has symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal with a density of 22.56 g/cm3 (0.815 lb/cu in) as defined by experimental X-ray crystallography. 191Ir and 193Ir are the only two naturally occurring isotopes of iridium, as well as the only stable isotopes; the latter is the more abundant. It is one of the most corrosion-resistant metals, even at temperatures as high as 2,000 °C (3,630 °F).

Group 9, by modern IUPAC numbering, is a group (column) of chemical elements in the d-block of the periodic table. Members of Group 9 include cobalt (Co), rhodium (Rh), iridium (Ir) and meitnerium (Mt). These elements are among the rarest of the transition metals.

Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is much more commonly encountered. The anhydrous salt has two polymorphs, α and β, which are brown and red colored respectively. More commonly encountered is the hygroscopic dark green trihydrate IrCl3(H2O)3 which is a common starting point for iridium chemistry.

The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an iridium-containing catalyst, such as the complex [Ir(CO)2I2]− (1).

Crabtree's catalyst is an organoiridium compound with the formula [C8H12IrP(C6H11)3C5H5N]PF6. It is a homogeneous catalyst for hydrogenation and hydrogen-transfer reactions, developed by Robert H. Crabtree. This air stable orange solid is commercially available and known for its directed hydrogenation to give trans stereoselectivity with respective of directing group.

A native metal is any metal that is found pure in its metallic form in nature. Metals that can be found as native deposits singly or in alloys include aluminium, antimony, arsenic, bismuth, cadmium, chromium, cobalt, indium, iron, manganese, molybdenum, nickel, niobium, rhenium, selenium, tantalum, tellurium, tin, titanium, tungsten, vanadium, and zinc, as well as the gold group and the platinum group. Among the alloys found in native state have been brass, bronze, pewter, German silver, osmiridium, electrum, white gold, silver-mercury amalgam, and gold-mercury amalgam.
Martin Arthur Bennett FRS is an Australian inorganic chemist. He gained recognition for studies on the co-ordination chemistry of tertiary phosphines, olefins, and acetylenes, and the relationship of their behaviour to homogeneous catalysis.

Organoiridium chemistry is the chemistry of organometallic compounds containing an iridium-carbon chemical bond. Organoiridium compounds are relevant to many important processes including olefin hydrogenation and the industrial synthesis of acetic acid. They are also of great academic interest because of the diversity of the reactions and their relevance to the synthesis of fine chemicals.
Bowieite is a rhodium-iridium-platinum sulfide mineral (Rh,Ir,Pt)2S3, found in platinum-alloy nuggets from Goodnews Bay, Alaska. It was named after the British scientist Stanley Bowie (1917–2008), in recognition of his work on identification of opaque minerals.

Pentamethylcyclopentadienyl iridium dichloride dimer is an organometallic compound with the formula [(C5(CH3)5IrCl2)]2, commonly abbreviated [Cp*IrCl2]2 This bright orange air-stable diamagnetic solid is a reagent in organometallic chemistry.
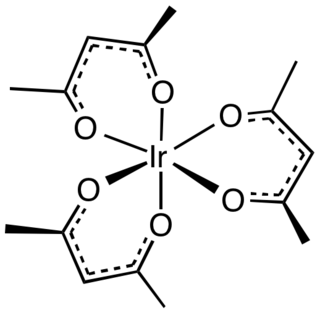
Iridium acetylacetonate is the iridium coordination complex with the formula Ir(O2C5H7)3, which is sometimes known as Ir(acac)3. The molecule has D3-symmetry. It is a yellow-orange solid that is soluble in organic solvents.

Cyclooctadiene iridium chloride dimer is an organoiridium compound with the formula [Ir(μ2-Cl)(COD)]2, where COD is the diene 1,5-cyclooctadiene (C8H12). It is an orange-red solid that is soluble in organic solvents. The complex is used as a precursor to other iridium complexes, some of which are used in homogeneous catalysis. The solid is air-stable but its solutions degrade in air.

Ammonium hexachloroiridate(IV) is the inorganic compound with the formula (NH4)2[IrCl6]. This dark red solid is the ammonium salt of the iridium(IV) complex [IrCl6]2−. It is a commercially important iridium compound one of the most common complexes of iridium(IV). A related but ill-defined compound is iridium tetrachloride, which is often used interchangeably.
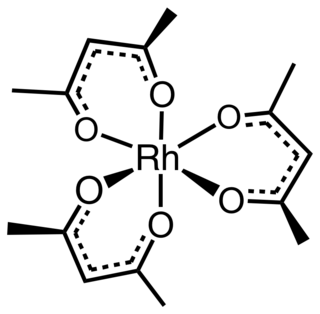
Rhodium acetylacetonate is the coordination complex with the formula Rh(C5H7O2)3, which is sometimes known as Rh(acac)3. The molecule has D3-symmetry. It is a yellow-orange solid that is soluble in organic solvents.

Rhodium(III) sulfide is the inorganic compound with the formula Rh2S3. It is an insoluble black solid, prepared by the heating a mixture of elemental rhodium and sulfur. Crystals can be grown by chemical vapor transport using bromine as the transporting agent. The structure consists of octahedral and tetrahedral Rh and S centers, respectively. No close Rh-Rh contacts are observed. Rh2Se3 and Ir2S3 adopt the same structure as Rh2S3.

Sodium hexachloroiridate(III) is an inorganic compound with the chemical formula Na3IrCl6.
Iridium compounds are compounds containing the element iridium (Ir). Iridium forms compounds in oxidation states between −3 and +9, but the most common oxidation states are +1, +2, +3, and +4. Well-characterized compounds containing iridium in the +6 oxidation state include IrF6 and the oxides Sr2MgIrO6 and Sr2CaIrO6. iridium(VIII) oxide was generated under matrix isolation conditions at 6 K in argon. The highest oxidation state (+9), which is also the highest recorded for any element, is found in gaseous [IrO4]+.
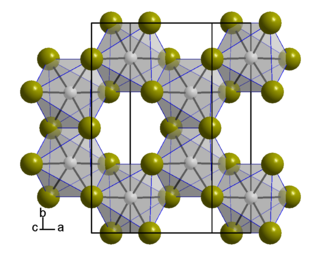
Iridium(III) bromide is a bromide of iridium(III), with the chemical formula of IrBr3.

Trimesityliridium is a pyramidal iridium(III) complex that crystallizes as a red-brown solid with the formula Ir(C9H11)3. It is most often used as an oxygen atom transfer catalyst in concert with oxotrimesityliridium, the product it forms readily when exposed to O2.















