
Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.

Beryllium fluoride is the inorganic compound with the formula BeF2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.

Copper monosulfide is a chemical compound of copper and sulfur. It was initially thought to occur in nature as the dark indigo blue mineral covellite. However, it was later shown to be rather a cuprous compound, formula Cu+3S(S2). CuS is a moderate conductor of electricity. A black colloidal precipitate of CuS is formed when hydrogen sulfide, H2S, is bubbled through solutions of Cu(II) salts. It is one of a number of binary compounds of copper and sulfur (see copper sulfide for an overview of this subject), and has attracted interest because of its potential uses in catalysis and photovoltaics.

Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a yellow-orange powder.

A uranate is a ternary oxide involving the element uranium in one of the oxidation states 4, 5 or 6. A typical chemical formula is MxUyOz, where M represents a cation. The uranium atom in uranates(VI) has two short collinear U–O bonds and either four or six more next nearest oxygen atoms. The structures are infinite lattice structures with the uranium atoms linked by bridging oxygen atoms.
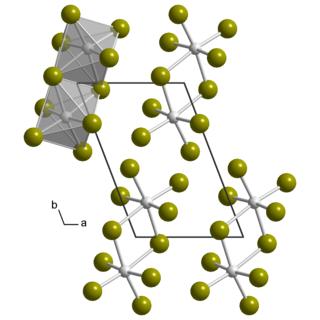
Uranium pentafluoride is the inorganic compound with the chemical formula UF5. It is a pale yellow paramagnetic solid. The compound has attracted interest because it is related to uranium hexafluoride, which is widely used to produce uranium fuel. It crystallizes in two polymorphs, called α- and β-UF5.

Aluminium sulfide is a chemical compound with the formula Al2S3. This colorless species has an interesting structural chemistry, existing in several forms. The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides. This can begin when the sulfide is exposed to the atmosphere. The hydrolysis reaction generates gaseous hydrogen sulfide (H2S).
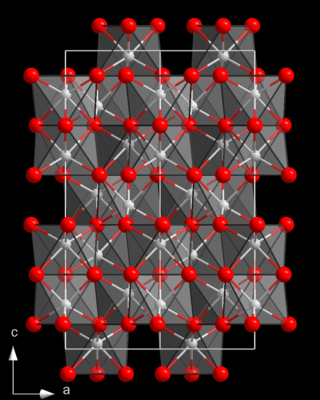
Titanium(III) oxide is the inorganic compound with the formula Ti2O3. A black semiconducting solid, it is prepared by reducing titanium dioxide with titanium metal at 1600 °C.

Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.

Few compounds of californium have been made and studied. The only californium ion that is stable in aqueous solutions is the californium(III) cation. The other two oxidation states are IV (strong oxidizing agents) and II (strong reducing agents). The element forms a water-soluble chloride, nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate or hydroxide. If problems of availability of the element could be overcome, then CfBr2 and CfI2 would likely be stable.

Berkelium forms a number of chemical compounds, where it normally exists in an oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid. Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halogens, chalcogens and pnictogens to form various binary compounds. Berkelium can also form several organometallic compounds.

Uranium pentachloride is an inorganic chemical compound composed of uranium in the +5 oxidation state and five chlorine atoms.
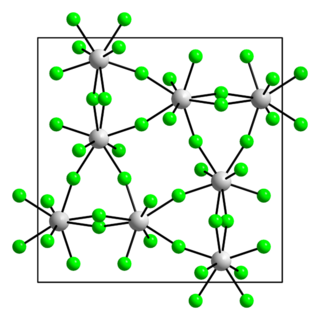
Uranium pentabromide is an inorganic chemical compound with the formula U2Br10.
In chemistry, molybdenum bronze is a generic name for certain mixed oxides of molybdenum with the generic formula A
xMo
yO
z where A may be hydrogen, an alkali metal cation (such as Li+, Na+, K+), and Tl+. These compounds form deeply coloured plate-like crystals with a metallic sheen, hence their name. These bronzes derive their metallic character from partially occupied 4d bands. The oxidation states in K0.28MoO3 are K+1, O2−, and Mo+5.72. MoO3 is an insulator, with an unfilled 4d band.
Phosphorus selenides are a relatively obscure group of compounds. There have been some studies of the phosphorus - selenium phase diagram and the glassy amorphous phases are reported. The compounds that have been reported are shown below. While some of phosphorus selenides are similar to their sulfide analogues, there are some new forms, molecular P2Se5 and the polymeric catena-[P4Se4]x. There is also some doubt about the existence of molecular P4Se10.

Gallium(III) sulfide, Ga2S3, is a compound of sulfur and gallium, that is a semiconductor that has applications in electronics and photonics.
Nitride fluorides containing nitride and fluoride ions with the formula NF4-. They can be electronically equivalent to a pair of oxide ions O24-. Nitride fluorides were discovered in 1996 by Lavalle et al. They heated diammonium technetium hexafluoride to 300 °C to yield TcNF. Another preparation is to heat a fluoride compound with a nitride compound in a solid state reaction. The fluorimido ion is F-N2- and is found in a rhenium compound.
Samarium compounds are compounds formed by the lanthanide metal samarium (Sm). In these compounds, samarium generally exhibits the +3 oxidation state, such as SmCl3, Sm(NO3)3 and Sm(C2O4)3. Compounds with samarium in the +2 oxidation state are also known, for example SmI2.
Neptunium compounds are compounds containg the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.
Americium compounds are compounds containing the element americium (Am). These compounds can form in the +2, +3, and +4, although the +3 oxidation state is the most common. The +5, +6 and +7 oxidation states have also been reported.













