Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel.

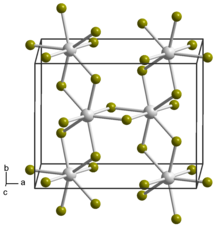
Uranium is a chemical element; it has symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactively decays by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 and uranium-235. Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite.

Carbon tetrabromide, CBr4, also known as tetrabromomethane, is a bromide of carbon. Both names are acceptable under IUPAC nomenclature.
Zirconium(IV) bromide is the inorganic compound with the formula ZrBr4. This colourless solid is the principal precursor to other Zr–Br compounds.
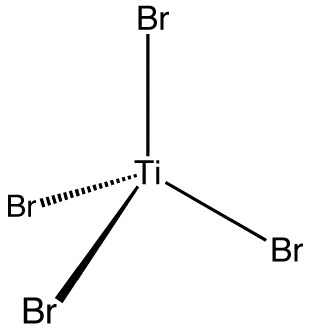
Titanium tetrabromide is the chemical compound with the formula TiBr4. It is the most volatile transition metal bromide. The properties of TiBr4 are an average of TiCl4 and TiI4. Some key properties of these four-coordinated Ti(IV) species are their high Lewis acidity and their high solubility in nonpolar organic solvents. TiBr4 is diamagnetic, reflecting the d0 configuration of the metal centre.

Tungsten(V) bromide is the inorganic compound with the empirical formula WBr5. The compound consists of bioctahedral structure, with two bridging bromide ligands, so its molecular formula is W2Br10.
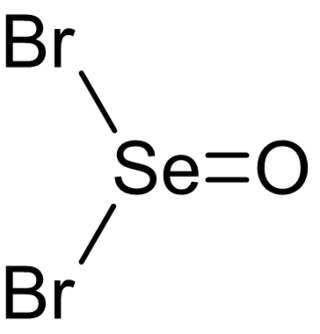
Selenium oxybromide (SeOBr2) is a selenium oxohalide chemical compound.

Silicon tetrabromide, also known as tetrabromosilane, is the inorganic compound with the formula SiBr4. This colorless liquid has a suffocating odor due to its tendency to hydrolyze with release of hydrogen bromide. The general properties of silicon tetrabromide closely resemble those of the more commonly used silicon tetrachloride.

1,1,2,2-Tetrabromoethane, or simply tetrabromoethane (TBE), is a halogenated hydrocarbon, chemical formula C2H2Br4. Although three bromine atoms may bind to one of the carbon atoms creating 1,1,1,2-tetrabromoethane this is not thermodynamically favorable, so in practice tetrabromoethane is equal to 1,1,2,2-tetrabromoethane, where each carbon atom binds two bromine atoms.
tetrabromide may refer to:
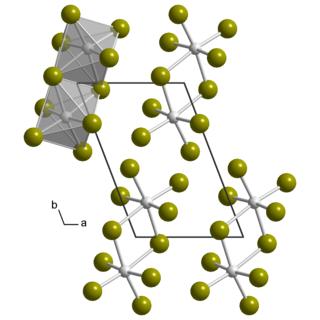
Uranium pentabromide is an inorganic chemical compound with the formula U2Br10.
Polonium dibromide (also known as polonium(II) bromide) is a chemical compound with the formula PoBr2. This salt is a purple-brown crystalline solid at room temperature. It sublimes (decomposing slightly) at 110 °C/30 μ and decomposes when melted in nitrogen gas at 270–280 °C.
Titanium(III) bromide is the inorganic compound with the formula TiBr3. It is a blue black paramagnetic solid with a reddish reflection. It has few applications, although it is a catalyst for the polymerization of alkenes.

Hafnium tetrabromide is the inorganic compound with the formula HfBr4. It is the most common bromide of hafnium. It is a colorless, diamagnetic moisture sensitive solid that sublimes in vacuum. It adopts a structure very similar to that of zirconium tetrabromide, featuring tetrahedral Hf centers, in contrast to the polymeric nature of hafnium tetrachloride.
Uranium bromide may refer to:

Selenium tetrabromide is an inorganic compound with a chemical formula SeBr4.

Germanium tetrabromide is the inorganic compound with the formula GeBr4. It is a colorless solid that melts near room temperature. It can be formed by treating solid germanium with bromine, or by treating a silicon-copper mixture with bromine:
Osmium tetrabromide is the inorganic compound with the formula OsBr4. A black solid, this compound can be produced by heating osmium tetrachloride and bromine under pressure.

Protactinium(IV) chloride is an inorganic compound. It is an actinide halide, composed of protactinium and chlorine. It is radioactive, and has the chemical formula of PaCl4. It is a chartreuse-coloured (yellowish-green) crystal of the tetragonal crystal system.