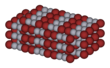
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest halogen, and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος ("stench"), referring to its sharp and disagreeable smell.

Mercury(I) chloride is the chemical compound with the formula Hg2Cl2. Also known as the mineral calomel (a rare mineral) or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury(I) compound. It is a component of reference electrodes in electrochemistry.
Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

Zinc bromide (ZnBr2) is an inorganic compound with the chemical formula ZnBr2. It is a colourless salt that shares many properties with zinc chloride (ZnCl2), namely a high solubility in water forming acidic solutions, and good solubility in organic solvents. It is hygroscopic and forms a dihydrate ZnBr2·2H2O.

Arsenic tribromide is the inorganic compound with the formula AsBr3. This pyramidal molecule is the only known binary arsenic bromide. AsBr3 is noteworthy for its very high refractive index of approximately 2.3. It also has a very high diamagnetic susceptibility. The compound exists as colourless deliquescent crystals that fume in moist air.
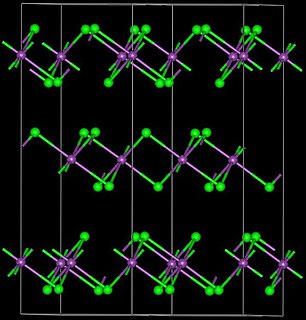
Yttrium(III) bromide is an inorganic compound with the chemical formula YBr3. It is a white solid. Anhydrous yttrium(III) bromide can be produced by reacting yttrium oxide or yttrium(III) bromide hydrate and ammonium bromide. The reaction proceeds via the intermediate (NH4)3YBr6. Another method is to react yttrium carbide (YC2) and elemental bromine. Yttrium(III) bromide can be reduced by yttrium metal to YBr or Y2Br3. It can react with osmium to produce Y4Br4Os.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.
Mercury(I) sulfide or mercurous sulfide is a hypothetical chemical compound of mercury and sulfur, with elemental formula Hg
2S. Its existence has been disputed; it may be stable below 0 °C or in suitable environments, but is unstable at room temperature, decomposing into metallic mercury and mercury(II) sulfide.

Copper(I) bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds and as a lasing medium in copper bromide lasers.

Mercury(I) sulfate, commonly called mercurous sulphate (UK) or mercurous sulfate (US) is the chemical compound Hg2SO4. Mercury(I) sulfate is a metallic compound that is a white, pale yellow or beige powder. It is a metallic salt of sulfuric acid formed by replacing both hydrogen atoms with mercury(I). It is highly toxic; it could be fatal if inhaled, ingested, or absorbed by skin.

Beryllium bromide is the chemical compound with the formula BeBr2. It is very hygroscopic and dissolves well in water. The compound is a polymer with tetrahedral Be centres.
Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a very toxic colourless gas described as having a "repulsive" odor. It is not widely encountered and has no commercial applications.
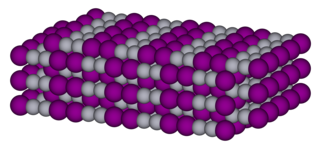
Mercury(I) iodide is a chemical compound of mercury and iodine. The chemical formula is Hg2I2. It is photosensitive and decomposes easily to mercury and HgI2.

Mercury(II) bromide or mercuric bromide is the inorganic compound with the formula HgBr2. This white solid is a laboratory reagent. Like all mercury salts, it is highly toxic.

Mercury(I) fluoride or mercurous fluoride is the chemical compound composed of mercury and fluorine with the formula Hg2F2. It consists of small yellow cubic crystals, which turn black when exposed to light.
Mercury polycations are polyatomic cations that contain only mercury atoms. The best known example is the Hg2+
2 ion, found in mercury(I) (mercurous) compounds. The existence of the metal–metal bond in Hg(I) compounds was established using X-ray studies in 1927 and Raman spectroscopy in 1934 making it one of the earliest, if not the first, metal–metal covalent bonds to be characterised.

Mercury(I) nitrate is a chemical compound with the formula Hg2(NO3)2. It was first discovered by Prafulla Chandra Ray It is used in the preparation of other mercury(I) compounds, and is toxic.
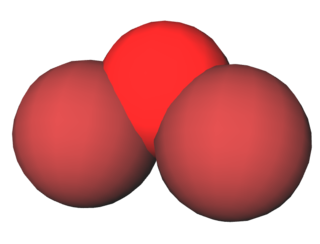
Dibromine monoxide is the chemical compound composed of bromine and oxygen with the formula Br2O. It is a dark brown solid which is stable below −40°C and is used in bromination reactions. It is similar to dichlorine monoxide, the monoxide of its halogen neighbor one period higher on the periodic table. The molecule is bent, with C2v molecular symmetry. The Br−O bond length is 1.85Å and the Br−O−Br bond angle is 112°, similar to dichlorine monoxide.

Dibromine pentoxide is the chemical compound composed of bromine and oxygen with the formula Br2O5. It is a colorless solid that is stable below −20 °C. It has the structure O2Br−O−BrO2, the Br−O−Br bond is bent with bond angle 121.2°. Each BrO3 group is pyramidal with the bromine atom at the apex.
Selenium tetrabromide is an inorganic compound with a chemical formula SeBr4.