 | |
 | |
| Names | |
|---|---|
| IUPAC name Mercury(I) sulfate | |
| Other names Mercurous sulfate | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.084 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
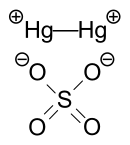
| Hg2SO4 | |
| Molar mass | 497.24 g/mol |
| Appearance | whitish-yellow crystals |
| Density | 7.56 g/cm3 |
| 0.051 g/100 mL (25 °C) 0.09 g/100 mL (100 °C) | |
Solubility product (Ksp) | 6.5×10−7 [1] |
| Solubility | soluble in dilute nitric acid, Insoluble in water, Soluble in hot sulfuric acid. |
| −123.0·10−6 cm3/mol | |
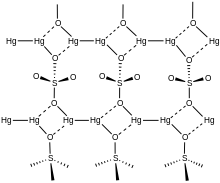
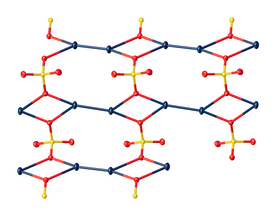
| Structure | |
| monoclinic | |
| Thermochemistry | |
Heat capacity (C) | 132 J·mol−1·K−1 [2] |
Std molar entropy (S⦵298) | 200.7 J·mol−1·K−1 |
Std enthalpy of formation (ΔfH⦵298) | -743.1 kJ·mol−1 |
| Related compounds | |
Other anions | Mercury(I) fluoride Mercury(I) chloride Mercury(I) bromide Mercury(I) iodide |
Other cations | Mercury(II) sulfate Cadmium sulfate Thallium(I) sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Mercury(I) sulfate, commonly called mercurous sulphate (UK) or mercurous sulfate (US) is the chemical compound Hg2SO4. [3] Mercury(I) sulfate is a metallic compound that is a white, pale yellow or beige powder. [4] It is a metallic salt of sulfuric acid formed by replacing both hydrogen atoms with mercury(I). It is highly toxic; it could be fatal if inhaled, ingested, or absorbed by skin.