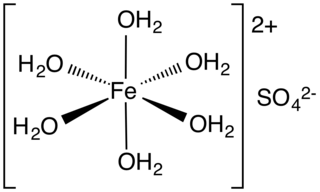
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula FeSO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known. The hydrated form is used medically to treat or prevent iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for hydrated sulfate minerals), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.

Magnesium sulfate or magnesium sulphate is a chemical compound, a salt with the formula MgSO4, consisting of magnesium cations Mg2+ (20.19% by mass) and sulfate anions SO2−4. It is a white crystalline solid, soluble in water but not in ethanol.

Copper(II) sulfate is an inorganic compound with the chemical formula CuSO4. It forms hydrates CuSO4·nH2O, where n can range from 1 to 7. The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate, while its anhydrous form is white. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper, and Roman vitriol. It exothermically dissolves in water to give the aquo complex [Cu(H2O)6]2+, which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The Cu(II)(H2O)4 centers are interconnected by sulfate anions to form chains.

Zinc sulfate describes a family of inorganic compounds with the formula ZnSO4(H2O)x. All are colorless solids. The most common form includes water of crystallization as the heptahydrate, with the formula ZnSO4·7H2O. As early as the 16th century it was prepared on the large scale, and was historically known as "white vitriol" (the name was used, for example, in 1620s by the collective writing under the pseudonym of Basil Valentine). Zinc sulfate and its hydrates are colourless solids.
Cadmium sulfate is the name of a series of related inorganic compounds with the formula CdSO4·xH2O. The most common form is the monohydrate CdSO4·H2O, but two other forms are known CdSO4·8⁄3H2O and the anhydrous salt (CdSO4). All salts are colourless and highly soluble in water.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Cobalt(II) chloride is an inorganic compound, a salt of cobalt and chlorine, with the formula CoCl
2. The compound forms several hydrates CoCl
2·nH
2O, for n = 1, 2, 6, and 9. Claims of the formation of tri- and tetrahydrates have not been confirmed. The anhydrous form is a blue crystalline solid; the dihydrate is purple and the hexahydrate is pink. Commercial samples are usually the hexahydrate, which is one of the most commonly used cobalt salts in the lab.

Vanadyl(IV) sulfate describes a collection of inorganic compounds of vanadium with the formula, VOSO4(H2O)x where 0 ≤ x ≤ 6. The pentahydrate is common. This hygroscopic blue solid is one of the most common sources of vanadium in the laboratory, reflecting its high stability. It features the vanadyl ion, VO2+, which has been called the "most stable diatomic ion".

Mercury(II) sulfate, commonly called mercuric sulfate, is the chemical compound HgSO4. It is an odorless salt that forms white granules or crystalline powder. In water, it separates into an insoluble basic sulfate with a yellow color and sulfuric acid.

Nickel(II) sulfate, or just nickel sulfate, usually refers to the inorganic compound with the formula NiSO4(H2O)6. This highly soluble blue green coloured salt is a common source of the Ni2+ ion for electroplating. Approximately 40,000 tonnes were produced in 2005.

Manganese(II) sulfate usually refers to the inorganic compound with the formula MnSO4·H2O. This pale pink deliquescent solid is a commercially significant manganese(II) salt. Approximately 260,000 tonnes of manganese(II) sulfate were produced worldwide in 2005. It is the precursor to manganese metal and many other chemical compounds. Manganese-deficient soil is remediated with this salt.

Cobalt nitrate is the inorganic compound with the formula Co(NO3)2.xH2O. It is cobalt(II)'s salt. The most common form is the hexahydrate Co(NO3)2·6H2O, which is a red-brown deliquescent salt that is soluble in water and other polar solvents.

Iron(III) sulfate (or ferric sulfate), is a family of inorganic compounds with the formula Fe2(SO4)3(H2O)n. A variety of hydrates are known, including the most commonly encountered form of "ferric sulfate". Solutions are used in dyeing as a mordant, and as a coagulant for industrial wastes. Solutions of ferric sulfate are also used in the processing of aluminum and steel.

Chromium(III) sulfate usually refers to the inorganic compounds with the formula Cr2(SO4)3.x(H2O), where x can range from 0 to 18. Additionally, ill-defined but commercially important "basic chromium sulfates" are known. These salts are usually either violet or green solids that are soluble in water. It is commonly used in tanning leather.
Cobalt poisoning is intoxication caused by excessive levels of cobalt in the body. Cobalt is an essential element for health in animals in minute amounts as a component of vitamin B12. A deficiency of cobalt, which is very rare, is also potentially lethal, leading to pernicious anemia.

Aplowite is a very rare mineral with the formula CoSO4•4H2O, a naturally occurring cobalt(II) sulfate tetrahydrate. It is the lower hydrate when compared to bieberite (heptahydrate) and moorhouseite (hexahydrate), and a higher hydrate when compared to cobaltkieserite (monohydrate). It occurs together with moorhouseite within efflorescences.
Moorhouseite is a rare mineral with the formula CoSO4•6H2O, a naturally occurring cobalt(II) sulfate hexahydrate. It is the lower-hydrate-equivalent of bieberite (heptahydrate) and aplowite (hexahydrate). It is also hydrated equivalent of cobaltkieserite. It occurs together with moorhouseite within efflorescences found in the Magnet Cove Barium Corporation mine in Walton, Nova Scotia, Canada.

Vanadium(II) sulfate describes a family of inorganic compounds with the formula VSO4(H2O)x where 0 ≤ x ≤ 7. The hexahydrate is most commonly encountered. It is a violet solid that dissolves in water to give air-sensitive solutions of the aquo complex. The salt is isomorphous with [Mg(H2O)6]SO4. Compared to the V–O bond length of 191 pm in [V(H2O)6]3+, the V–O distance is 212 pm in the [V(H2O)6]SO4. This nearly 10% elongation reflects the effect of the lower charge, hence weakened electrostatic attraction.

The Nickel oxyacid salts are a class of chemical compounds of nickel with an oxyacid. The compounds include a number of minerals and industrially important nickel compounds.
Gallium(III) sulfate refers to the chemical compound, a salt, with the formula Ga2(SO4)3, or its hydrates Ga2(SO4)3·xH2O. Gallium metal dissolves in sulfuric acid to form solutions containing [Ga(OH2)6]3+ and SO42− ions. The octadecahydrate Ga2(SO4)3·18H2O crystallises from these solutions at room temperature. This hydrate loses water in stages when heated, forming the anhydrate Ga2(SO4)3 above 150 °C and completely above 310 °C. Anhydrous Ga2(SO4)3 is isostructural with iron(III) sulfate, crystallizing in the rhombohedral space group R3.






















