 | |
 | |
| Names | |
|---|---|
| IUPAC name Disulfuric acid [1] | |
| Other names Pyrosulfuric acid, Oleum | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.069 |
| EC Number |
|
| MeSH | Pyrosulfuric+acid |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
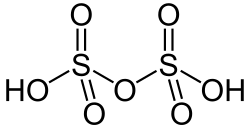
| H2O7S2 | |
| Molar mass | 178.13 g·mol−1 |
| Appearance | colorless |
| Melting point | 36 °C (97 °F; 309 K) |
| Acidity (pKa) | 2.5 (20 °C; in conc. H2SO4) [2] |
| Conjugate base | Disulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Disulfuric acid (alternative spelling disulphuric acid) or pyrosulfuric acid (alternative spelling pyrosulphuric acid), also named oleum, is a sulfur oxoacid. [3] It is a major constituent of fuming sulfuric acid, oleum, and this is how most chemists encounter it. As confirmed by X-ray crystallography, the molecule consists of a pair of SO2(OH) groups joined by an oxygen atom, [4] giving a molecular formula of H2O7S2.