Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.
In chemistry, a zwitterion, also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively and negatively charged functional groups. With amino acids, for example, in solution a chemical equilibrium will be established between the "parent" molecule and the zwitterion.

A Belousov–Zhabotinsky reaction, or BZ reaction, is one of a class of reactions that serve as a classical example of non-equilibrium thermodynamics, resulting in the establishment of a nonlinear chemical oscillator. The only common element in these oscillators is the inclusion of bromine and an acid. The reactions are important to theoretical chemistry in that they show that chemical reactions do not have to be dominated by equilibrium thermodynamic behavior. These reactions are far from equilibrium and remain so for a significant length of time and evolve chaotically. In this sense, they provide an interesting chemical model of nonequilibrium biological phenomena; as such, mathematical models and simulations of the BZ reactions themselves are of theoretical interest, showing phenomenon as noise-induced order.
Cubane is a synthetic hydrocarbon compound with the formula C8H8, and that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.
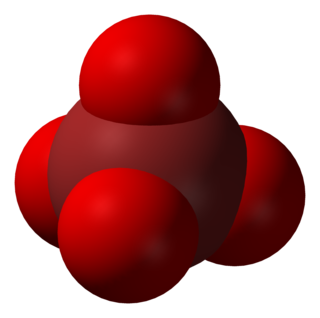
In chemistry, the perbromate ion is the anion having the chemical formula BrO−
4. It is an oxyanion of bromine, the conjugate base of perbromic acid, in which bromine has the oxidation state +7. Unlike its chlorine and iodine analogs, it is difficult to synthesize. It has tetrahedral molecular geometry.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.
In chemistry, a strong electrolyte is a solute that completely, or almost completely, ionizes or dissociates in a solution. These ions are good conductors of electric current in the solution.
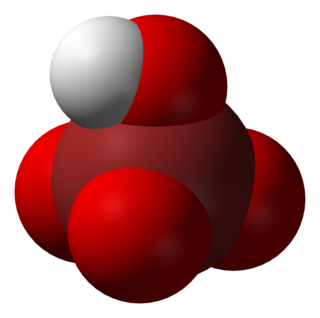
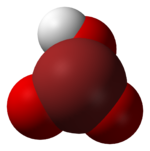
Perbromic acid is the inorganic compound with the formula HBrO4. Perbromic acid is characterized as a colorless liquid which has no characteristic scent. It is an oxoacid of bromine, with an oxidation state of 7+. Perbromic acid is a strong acid and strongly oxidizing, though dilute perbromic acid solutions are slow oxidizing agents. It is the most unstable of the halogen(VII) oxoacids. It decomposes rapidly on standing to bromic acid and oxygen, which releases toxic brown bromine vapors. It can be used in the synthesis of perbromate salts, by reacting with a base.

The Briggs–Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking colour changes: the freshly prepared colourless solution slowly turns an amber colour, then suddenly changes to a very dark blue. This slowly fades to colourless and the process repeats, about ten times in the most popular formulation, before ending as a dark blue liquid smelling strongly of iodine.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.

Barium bromide is the chemical compound with the formula BaBr2. It is ionic and hygroscopic in nature.

Bromous acid is the inorganic compound with the formula of HBrO2. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.

Chlorine perchlorate is a chemical compound with the formula Cl2O4. This chlorine oxide is an asymmetric oxide, with one chlorine atom in +1 oxidation state and the other +7, with proper formula ClOClO3. It is produced by the photodimerization of chlorine dioxide (ClO2) at room temperature by 436 nm ultraviolet light:

A chemical oscillator is a complex mixture of reacting chemical compounds in which the concentration of one or more components exhibits periodic changes. They are a class of reactions that serve as an example of non-equilibrium thermodynamics with far-from-equilibrium behavior. The reactions are theoretically important in that they show that chemical reactions do not have to be dominated by equilibrium thermodynamic behavior.
Barium perchlorate is a powerful oxidizing agent, with the formula Ba(ClO4)2. It is used in the pyrotechnic industry.
Barium permanganate is a chemical compound, with the formula Ba(MnO4)2. It forms violet to brown crystals that are sparingly soluble in water.

Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water.
A halous acid, also known as a halogenous acid, is an oxyacid consisting of a halogen atom in the +3 oxidation state single-bonded to a hydroxyl group and double-bonded to an oxygen atom. Examples include chlorous acid, bromous acid, and iodous acid. The conjugate base is a halite.