
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen ion, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.

In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.

In chemistry, there are three definitions in common use of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.
In chemistry, an amphoteric compound is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was first developed by Johannes Nicolaus Brønsted and Thomas Martin Lowry independently in 1923. The basic concept of this theory is that when an acid and a base react with each other, the acid forms its conjugate base, and the base forms its conjugate acid by exchange of a proton (the hydrogen cation, or H+). This theory generalises the Arrhenius theory.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.

Tellurium dioxide (TeO2) is a solid oxide of tellurium. It is encountered in two different forms, the yellow orthorhombic mineral tellurite, β-TeO2, and the synthetic, colourless tetragonal (paratellurite), α-TeO2. Most of the information regarding reaction chemistry has been obtained in studies involving paratellurite, α-TeO2.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

In chemistry, tellurate is a compound containing an oxyanion of tellurium where tellurium has an oxidation number of +6. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central tellurium atom.

Telluric acid, or more accurately orthotelluric acid, is a chemical compound with the formula Te(OH)6, often written as H6TeO6. It is a white crystalline solid made up of octahedral Te(OH)6 molecules which persist in aqueous solution. In the solid state, there are two forms, rhombohedral and monoclinic, and both contain octahedral Te(OH)6 molecules, containing one hexavalent tellurium (Te) atom in the +6 oxidation state, attached to six hydroxyl (–OH) groups, thus, it can be called tellurium(VI) hydroxide. Telluric acid is a weak acid which is dibasic, forming tellurate salts with strong bases and hydrogen tellurate salts with weaker bases or upon hydrolysis of tellurates in water. It is used as tellurium-source in the synthesis of oxidation catalysts.

Arsenic acid or arsoric acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO(OH)3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but is only found in solution, where it is largely ionized. Its hemihydrate form (2H3AsO4·H2O) does form stable crystals. Crystalline samples dehydrate with condensation at 100 °C.

Arsenous acid (or arsenious acid) is the inorganic compound with the formula H3AsO3. It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As(OH)3.
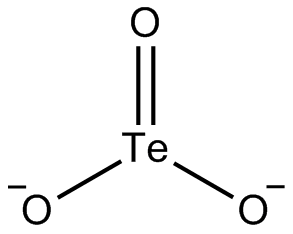
Tellurite is a oxyanion of tellurium with the formula TeO2−
3. It is the ion of tellurous acid, and is chemically related to tellurium dioxide, whose mineral appearance also bears the name tellurite. Tellurites are typically colorless or white salts, which in some ways are comparable to sulfite.

Sodium tellurite is an inorganic tellurium compound with formula Na2TeO3. It is a water-soluble white solid and a weak reducing agent. Sodium tellurite is an intermediate in the extraction of the element, tellurium; it is a product obtained from anode slimes and is a precursor to tellurium.

Hydrogen telluride is the inorganic compound with the formula H2Te. A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas. Although unstable in ambient air, the gas can exist long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations. Most compounds with Te–H bonds (tellurols) are unstable with respect to loss of H2. H2Te is chemically and structurally similar to hydrogen selenide, both are acidic. The H–Te–H angle is about 90°. Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.

Polonium dioxide (also known as polonium(IV) oxide) is a chemical compound with the formula PoO2. It is one of three oxides of polonium, the other two being polonium monoxide (PoO) and polonium trioxide (PoO3). It is a pale yellow crystalline solid at room temperature. Under lowered pressure (such as a vacuum), it decomposes into elemental polonium and oxygen at 500 °C. It is the most stable oxide of polonium and is an interchalcogen.
Tellurium compounds are compounds containing the element tellurium (Te). Tellurium belongs to the chalcogen family of elements on the periodic table, which also includes oxygen, sulfur, selenium and polonium: Tellurium and selenium compounds are similar. Tellurium exhibits the oxidation states −2, +2, +4 and +6, with +4 being most common.
















