Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.

Redox is a type of chemical reaction in which the oxidation states of atoms are changed. Redox reactions are characterized by the actual or formal transfer of electrons between chemical species, most often with one species undergoing oxidation while another species undergoes reduction. The chemical species from which the electron is removed is said to have been oxidized, while the chemical species to which the electron is added is said to have been reduced. In other words:

In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances which react with acids as originally proposed by G.-F. Rouelle in the mid-18th century.

An electrolytic cell is an electrochemical cell that uses electrical energy to drive a non-spontaneous redox reaction. It is often used to decompose chemical compounds, in a process called electrolysis—the Greek word lysis means to break up. Important examples of electrolysis are the decomposition of water into hydrogen and oxygen, and bauxite into aluminium and other chemicals. Electroplating is done using an electrolytic cell. Electrolysis is a technique that uses a direct electric current (DC).

Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula Na2S2O3.xH2O. Typically it is available as the white or colorless pentahydrate, Na2S2O3·5H2O. The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water.
The telluride ion is the anion Te2− and its derivatives. It is analogous to the other chalcogenide anions, the lighter O2−, S2−, and Se2−, and the heavier Po2−.

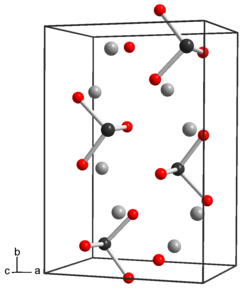
Tellurium dioxide (TeO2) is a solid oxide of tellurium. It is encountered in two different forms, the yellow orthorhombic mineral tellurite, β-TeO2, and the synthetic, colourless tetragonal (paratellurite), α-TeO2. Most of the information regarding reaction chemistry has been obtained in studies involving paratellurite, α-TeO2.

In chemistry tellurate is a compound containing an oxyanion of tellurium where tellurium has an oxidation number of +6. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central tellurium atom.

Telluric acid is a chemical compound with the formula Te(OH)6. It is a white solid made up of octahedral Te(OH)6 molecules which persist in aqueous solution. There are two forms, rhombohedral and monoclinic, and both contain octahedral Te(OH)6 molecules. Telluric acid is a weak acid which is dibasic, forming tellurate salts with strong bases and hydrogen tellurate salts with weaker bases or upon hydrolysis of tellurates in water. It is used as tellurium-source in the synthesis of oxidation catalysts.
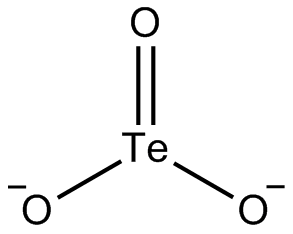
The tellurite ion is TeO2−
3. A tellurite (compound), for example sodium tellurite, is a compound that contains this ion. They are typically colorless or white salts, which in some ways are comparable to sulfite. A mineral with the formula TeO2 is called tellurite.

Tellurous acid is an inorganic compound with the formula H2TeO3. It is the oxoacid of tellurium(IV). This compound is not well characterized. An alternative way of writing its formula is (HO)2TeO. In principle, tellurous acid would form by treatment of tellurium dioxide with water, that is by hydrolysis. The related conjugate base is well known in the form of several salts such as potassium hydrogen tellurite, KHTeO3.

Sodium sulfide is the chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts are colorless solids. They are water-soluble, giving strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, which smells like rotten eggs.

Sodium phosphide is the inorganic compound with the formula Na3P. It is a black solid. It is often described as Na+ salt of the P3− anion. Na3P is a source of the highly reactive phosphide anion. It should not be confused with sodium phosphate, Na3PO4.
Hydrogen telluride (tellane) is the inorganic compound with the formula H2Te. A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas. Although unstable in ambient air, the gas can exist at very low concentrations long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations. Most compounds with Te–H bonds (tellurols) are unstable with respect to loss of H2. H2Te is chemically and structurally similar to hydrogen selenide, both are acidic. The H–Te–H angle is about 90°. Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.
Sodium telluride is the chemical compound with the formula Na2Te. This salt is the conjugate base of the thermally unstable acid hydrogen telluride, but it is usually prepared by reduction of tellurium with sodium. Na2Te is a challenging material to handle because it is very sensitive to air. Air oxidizes it initially to polytellurides, which have the formula Na2Tex (x > 1), and ultimately Te metal. Samples of Na2Te, which are colourless when absolutely pure, generally appear purple or dark gray due to the effects of air oxidation.
Hyponitrous acid is a chemical compound with formula H
2N
2O
2 or HON=NOH. It is an isomer of nitramide, H2N−NO2.
Organotellurium chemistry describes the synthesis and properties of chemical compounds containing a carbon-tellurium chemical bond. Organotellurium chemistry is a lightly studied area, in part because of the few applications.
Thiocarbonate describes a family of anions with the general chemical formula CS
3−xO2−
x. Like the carbonate dianion, the thiocarbonates are planar, with carbon at the center. The average bond order from C to S or O is 1+1⁄3. The state of protonation is usually not specified. These anions are good nucleophiles and good ligands.

Sodium bismuthate is an inorganic compound, and a strong oxidiser with chemical formula NaBiO3. It is somewhat hygroscopic, but not soluble in cold water, which can be convenient since the reagent can be easily removed after the reaction. It is one of the few water insoluble sodium salts. Commercial samples may be a mixture of bismuth(V) oxide, sodium carbonate and sodium peroxide.