
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+
and hydroxide anions OH−
.

Sodium chloride, commonly known as salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form of table salt, it is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. A second major application of sodium chloride is de-icing of roadways in sub-freezing weather.

Sodium carbonate, Na2CO3·10H2O, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process.

Sodium hypochlorite is a chemical compound with the formula NaOCl or NaClO, comprising a sodium cation and a hypochlorite anion. It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate NaOCl·5H
2O, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated.

Sodium percarbonate is a chemical substance with formula Na
2H
3CO
6. It is an adduct of sodium carbonate and hydrogen peroxide whose formula is more properly written as 2 Na
2CO
3 · 3 H
2O
2. It is a colorless, crystalline, hygroscopic and water-soluble solid. It is sometimes abbreviated as SPC. It contains 32.5% by weight of hydrogen peroxide.

Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (x = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form.

Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2SO3. A white, water-soluble solid, it is used commercially as an antioxidant and preservative. A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air.

Drano is an American brand of drain cleaner that is manufactured by S. C. Johnson & Son.

Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate (anhydrous) is a white crystalline solid having a formula variously given as NaAlO2, NaAl(OH)4 (hydrated), Na2O·Al2O3, or Na2Al2O4. Commercial sodium aluminate is available as a solution or a solid.
Other related compounds, sometimes called sodium aluminate, prepared by reaction of Na2O and Al2O3 are Na5AlO4 which contains discrete AlO45− anions, Na7Al3O8 and Na17Al5O16 which contain complex polymeric anions, and NaAl11O17, once mistakenly believed to be β-alumina, a phase of aluminium oxide.

Sodium periodate is an inorganic salt, composed of a sodium cation and the periodate anion. It may also be regarded as the sodium salt of periodic acid. Like many periodates, it can exist in two different forms: sodium metaperiodate (formula NaIO4) and sodium orthoperiodate (normally Na2H3IO6, but sometimes the fully reacted salt Na5IO6). Both salts are useful oxidising agents.
Sodium perborate is chemical compound whose chemical formula may be written NaH
2BO
4, Na
2H
4B
2O
8, or, more properly, [Na+
]
2·[B
2O
4(OH)
4]2−
. Its name is sometimes abbreviated as PBS.

Sodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder.

Sodium permanganate is the inorganic compound with the formula NaMnO4. It is closely related to the more commonly encountered potassium permanganate, but it is generally less desirable, because it is more expensive to produce. It is mainly available as the monohydrate. This salt absorbs water from the atmosphere and has a low melting point. Being about 15 times more soluble than KMnO4, sodium permanganate finds some applications where very high concentrations of MnO4− are sought.
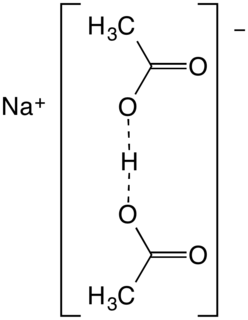
Sodium diacetate is a compound with formula NaH(C
2H
3O
2)
2. It is a salt of acetic acid. It is a colorless solid that is used in seasonings and as an antimicrobial agent.
Praseodymium(III) sulfate is a praseodymium compound with formula Pr2(SO4)3. It is an odourless whitish-green crystalline compound. The anhydrous substance readily absorbs water forming pentahydrate and octahydrate.
The purpose of a mineralizer is to facilitate the transport of insoluble “nutrient” to a seed crystal by means of a reversible chemical reaction. Over time, the seed crystal accumulates the material that was once in the nutrient and grows. Mineralizers are additives that aid the solubilization of the nutrient solid. When used in small quantities, mineralizers function as catalysts. Typically, a more stable solid is crystallized from a solution that consists of a less stable solid and a solvent. The process is done by dissolution-precipitation or crystallization process.

Thorium(IV) nitrate is a chemical compound with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.

Sodium orthosilicate is the chemical compound with the molecular formula Na
4SiO
4. It is one of the sodium silicates, specifically an orthosilicate, formally a salt of the unstable orthosilicic acid H
4SiO
4.

Sodium hydrogenoxalate is salt of formula NaHC
2O
4, consisting of sodium cations Na+
and hydrogenoxalate anions HC
2O−
4 or -. The anion can be described as the result of removing one hydrogen ion H+
from oxalic acid H
2C
2O
4, or adding one to the oxalate anion C
2O2−
4.

Neodymium(III) sulfate is a salt of the rare earth metal neodymium that has the formula Nd2(SO4)3. It forms multiple hydrates, the octa-, penta-, and the dihydrate, which the octahydrate is the most common. This compound has a retrograde solubility, unlike other compounds, its solubility decreases with increasing temperature. This compound is used in glass for extremely powerful lasers.


















