
An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
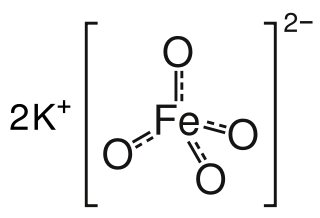
Potassium ferrate is an inorganic compound with the formula K2FeO4. It is the potassium salt of ferric acid. Potassium ferrate is a powerful oxidizing agent with applications in green chemistry, organic synthesis, and cathode technology.
Sodium bromide is an inorganic compound with the formula NaBr. It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion and has many applications.
The sodium fusion test, or Lassaigne's test, is used in elemental analysis for the qualitative determination of the presence of foreign elements, namely halogens, nitrogen, and sulfur, in an organic compound. It was developed by J. L. Lassaigne.

A permanganate is a chemical compound with the manganate(VII) ion, MnO−
4, the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition metal ion with a tetrahedral structure. Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media. The exact chemical reaction depends on the carbon-containing reactants present and the oxidant used. For example, trichloroethane (C2H3Cl3) is oxidised by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).

Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate (anhydrous) is a white crystalline solid having a formula variously given as NaAlO2, NaAl(OH)4 (hydrated), Na2O·Al2O3, or Na2Al2O4. Commercial sodium aluminate is available as a solution or a solid.
Other related compounds, sometimes called sodium aluminate, prepared by reaction of Na2O and Al2O3 are Na5AlO4 which contains discrete AlO45− anions, Na7Al3O8 and Na17Al5O16 which contain complex polymeric anions, and NaAl11O17, once mistakenly believed to be β-alumina, a phase of aluminium oxide.
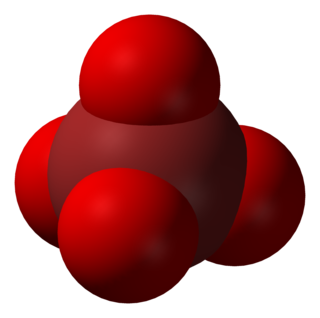
In chemistry, the perbromate ion is the anion having the chemical formula BrO−
4. It is an oxyanion of bromine, the conjugate base of perbromic acid, in which bromine has the oxidation state +7. Unlike its chlorine and iodine analogs, it is difficult to synthesize. It has tetrahedral molecular geometry.
In chemistry, an aluminate is a compound containing an oxyanion of aluminium, such as sodium aluminate. In the naming of inorganic compounds, it is a suffix that indicates a polyatomic anion with a central aluminium atom.
In chemistry, a strong electrolyte is a solute that completely, or almost completely, ionizes or dissociates in a solution. These ions are good conductors of electric current in the solution.

Sodium molybdate, Na2MoO4, is useful as a source of molybdenum. This white, crystalline salt is often encountered as the dihydrate, Na2MoO4·2H2O.
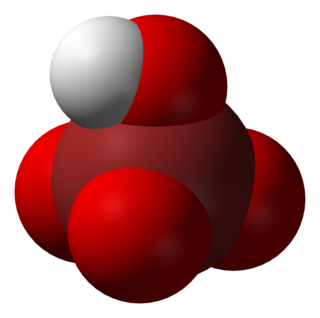
Perbromic acid is the inorganic compound with the formula HBrO4. Perbromic acid is characterized as a colorless liquid which has no characteristic scent. It is an oxoacid of bromine, with an oxidation state of +7. Perbromic acid is a strong acid and strongly oxidizing, though dilute perbromic acid solutions are slow oxidizing agents. It is the most unstable of the halogen(VII) oxoacids. It decomposes rapidly on standing to bromic acid and oxygen, which releases toxic brown bromine vapors. It can be used in the synthesis of perbromate salts, by reacting with a base.

Sodium oxalate, or disodium oxalate, is a chemical compound with the chemical formula Na2C2O4. It is the sodium salt of oxalic acid. It contains sodium cations Na+ and oxalate anions C2O2−4. It is a white, crystalline, odorless solid, that decomposes above 290 °C.

Sodium perrhenate (also known as sodium rhenate(VII)) is the inorganic compound with the formula NaReO4. It is a white salt that is soluble in water. It is a common precursor to other rhenium compounds. Its structure resembles that of sodium perchlorate and sodium permanganate.

15-Crown-5 is a crown ether with the formula (C2H4O)5. It is a cyclic pentamer of ethylene oxide that forms complex with various cations, including sodium (Na+) and potassium (K+); however, it is complementary to Na+ and thus has a higher selectivity for Na+ ions.

Bromine can form several different oxides:

Iron(II) molybdate is an inorganic compound with the chemical formula FeMoO4.
The periodatonickelates are a series of anions and salts of nickel complexed to the periodate anion. The most important of these salts are the diperiodatonickelates, in which nickel exhibits the +4 oxidation state: these are powerful oxidising agents, capable of oxidising bromate to perbromate.

Potassium perbromate is the chemical compound composed of the potassium ion and the perbromate ion, with the chemical formula KBrO4.













