
An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Trisodium phosphate (TSP) is the inorganic compound with the chemical formula Na3PO4. It is a white, granular or crystalline solid, highly soluble in water, producing an alkaline solution. TSP is used as a cleaning agent, builder, lubricant, food additive, stain remover, and degreaser.

Tetrasodium pyrophosphate, also called sodium pyrophosphate, tetrasodium phosphate or TSPP, is an inorganic compound with the formula Na4P2O7. As a salt, it is a white, water-soluble solid. It is composed of pyrophosphate anion and sodium ions. Toxicity is approximately twice that of table salt when ingested orally. Also known is the decahydrate Na4P2O7 · 10(H2O).
Acid salts are a class of salts that produce an acidic solution after being dissolved in a solvent. Its formation as a substance has a greater electrical conductivity than that of the pure solvent. An acidic solution formed by acid salt is made during partial neutralization of diprotic or polyprotic acids. A half-neutralization occurs due to the remaining of replaceable hydrogen atoms from the partial dissociation of weak acids that have not been reacted with hydroxide ions to create water molecules.
The arsenate is an ion with the chemical formula AsO3−4. Bonding in arsenate consists of a central arsenic atom, with oxidation state +5, double bonded to one oxygen atom and single bonded to a further three oxygen atoms. The four oxygen atoms orient around the arsenic atom in a tetrahedral geometry. Resonance disperses the ion's −3 charge across all four oxygen atoms.

Arsenic acid or trihydrogen arsenate is the chemical compound with the formula H3AsO4. More descriptively written as AsO(OH)3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but is only found in solution, where it is largely ionized. Its hemihydrate form (2H3AsO4·H2O) does form stable crystals. Crystalline samples dehydrate with condensation at 100 °C.
In chemistry, an arsenite is a chemical compound containing an arsenic oxyanion where arsenic has oxidation state +3. Note that in fields that commonly deal with groundwater chemistry, arsenite is used generically to identify soluble AsIII anions. IUPAC have recommended that arsenite compounds are to be named as arsenate(III), for example ortho-arsenite is called trioxidoarsenate(III). Ortho-arsenite contrasts to the corresponding anions of the lighter members of group 15, phosphite which has the structure HPO2−3 and nitrite, NO−2 which is bent.
Sodium perborate is chemical compound whose chemical formula may be written NaH2BO4, Na2H4B2O8, or, more properly, [Na+]2[B2O4(OH)4]2−. Its name is sometimes abbreviated as PBS.

Calcium arsenate is the inorganic compound with the formula Ca3(AsO4)2. A colourless salt, it was originally used as a pesticide and as a germicide. It is highly soluble in water, in contrast to lead arsenate, which makes it more toxic. The minerals rauenthalite Ca3(AsO4)2·10H2O and phaunouxite Ca3(AsO4)2·11H2O are hydrates of calcium arsenate.
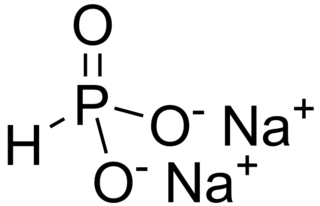
Disodium hydrogen phosphite is the name for inorganic compounds with the formula Na2HPO3•(H2O)x. The commonly encountered salt is the pentahydrate. A derivative of phosphorous acid (HP(O)(OH)2), it contains the anion HPO32−. Its common name suggests that it contains an acidic hydrogen atom, as in sodium hydrogen carbonate. However, this name is misleading as the hydrogen atom is not acidic, being bonded to phosphorus rather than oxygen. The salt has reducing properties. It is white or colorless solid, and is little studied.

Disodium phosphate (DSP), or disodium hydrogen phosphate, or sodium phosphate dibasic, is the inorganic compound with the formula Na2HPO4. It is one of several sodium phosphates. The salt is known in anhydrous form as well as forms with 2, 7, 8, and 12 hydrates. All are water-soluble white powders; the anhydrous salt being hygroscopic.

Sodium arsenate is the inorganic compound with the formula Na3AsO4. Related salts are also called sodium arsenate, including Na2HAsO4 (disodium hydrogen arsenate) and NaH2AsO4 (sodium dihydrogen arsenate). The trisodium salt is a white or colourless solid that is highly toxic. It is usually handled as the dodecahydrate Na3AsO4.12H2O.

Disodium methyl arsonate (DSMA) is the organoarsenic compound with the formula CH3AsO3Na2. It is a colorless, water-soluble solid derived from methanearsonic acid. It is used as a herbicide. Tradenames include Metharsinat, Arrhenal, Disomear, Metharsan, Stenosine, Tonarsan, Tonarsin, Arsinyl, Arsynal, and Diarsen.
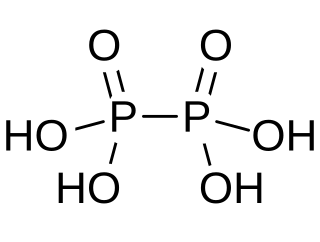
Hypophosphoric acid is a mineral acid with the formula H4P2O6, with phosphorus in a formal oxidation state of +4. In the solid state it is present as the dihydrate, H4P2O6·2H2O. In hypophosphoric acid the phosphorus atoms are identical and joined directly with a P−P bond. Isohypophosphoric acid is a structural isomer of hypophosphoric acid in which one phosphorus has a hydrogen directedly bonded to it and that phosphorus atom is linked to the other one by an oxygen bridge to give a phosphorous acid/phosphoric acid mixed anhydride. The two phosphorus atoms are in the +3 and +5 oxidation states, respectively.

Sodium dihydrogen arsenate is the inorganic compound with the formula NaH2AsO4. Related salts are also called sodium arsenate, including Na2HAsO4 (disodium hydrogen arsenate) and NaH2AsO4 (sodium dihydrogen arsenate). Sodium dihydrogen arsenate is a colorless solid that is highly toxic.
Nitroxylic acid or hydronitrous acid is an unstable reduced oxonitrogen acid. It has formula H4N2O4 containing nitrogen in the +2 oxidation state. The corresponding anion called nitroxylate is N
2O4−
4 or NO2−
2.
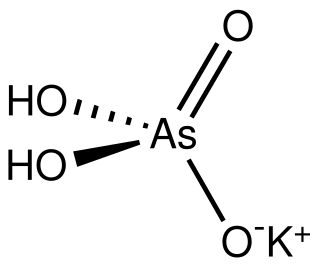
Monopotassium arsenate is the inorganic compound with the formula KH2AsO4. A white solid, this salt is used to prepared other arsenic-containing compounds, mainly pesticides. It is prepared by calcining arsenic oxide and potassium nitrate, followed by extraction with water.

Praseodymium arsenate is the arsenate salt of praseodymium, with the chemical formula of PrAsO4. It has good thermal stability. Its ferroelectric transition temperature is 52°C.
Disodium enneaborate is the traditional name for a salt of sodium, boron, oxygen, and hydrogen, with elemental formula Na2B9H22O20 or Na2B9O9·11H2O. It is the sodium borate with the highest boron/sodium ratio.

















