Sodium bromide is an inorganic compound with the formula NaBr. It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion and has many applications.

Sodium perchlorate is an inorganic compound with the chemical formula NaClO4. It consists of sodium cations Na+ and perchlorate anions ClO−4. It is a white crystalline, hygroscopic solid that is highly soluble in water and ethanol. It is usually encountered as sodium perchlorate monohydrate NaClO4·H2O. The compound is noteworthy as the most water-soluble of the common perchlorate salts.

Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate (anhydrous) is a white crystalline solid having a formula variously given as NaAlO2, NaAl(OH)4 (hydrated), Na2O·Al2O3, or Na2Al2O4. Commercial sodium aluminate is available as a solution or a solid.
Other related compounds, sometimes called sodium aluminate, prepared by reaction of Na2O and Al2O3 are Na5AlO4 which contains discrete AlO45− anions, Na7Al3O8 and Na17Al5O16 which contain complex polymeric anions, and NaAl11O17, once mistakenly believed to be β-alumina, a phase of aluminium oxide.
Lead hydrogen arsenate, also called lead arsenate, acid lead arsenate or LA, chemical formula PbHAsO4, is an inorganic insecticide used primarily against the potato beetle. Lead arsenate was the most extensively used arsenical insecticide. Two principal formulations of lead arsenate were marketed: basic lead arsenate (Pb5OH(AsO4)3, CASN: 1327-31-7) and acid lead arsenate (PbHAsO4).
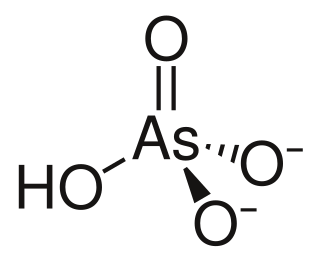
The arsenate is an ion with the chemical formula AsO3−4. Bonding in arsenate consists of a central arsenic atom, with oxidation state +5, double bonded to one oxygen atom and single bonded to a further three oxygen atoms. The four oxygen atoms orient around the arsenic atom in a tetrahedral geometry. Resonance disperses the ion's −3 charge across all four oxygen atoms.

Arsenic acid or arsoric acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO(OH)3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but is only found in solution, where it is largely ionized. Its hemihydrate form (2H3AsO4·H2O) does form stable crystals. Crystalline samples dehydrate with condensation at 100 °C.
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications.

Sodium arsenite usually refers to the inorganic compound with the formula NaAsO2. Also called sodium meta-arsenite, it is an inorganic polymer consisting of the infinite chains [AsO2]n−
n associated with sodium cations, Na+. The polymer backbone has the connectivity -O-As(O−)-.backbone. Sodium ortho-arsenite is Na3AsO3. Both compounds are colourless solids. A mixture of sodium meta-arsenite and sodium ortho-arsenite is produced by treating arsenic trioxide with sodium carbonate or sodium hydroxide. Sodium arsenite is amorphous, typically being obtained as a powder or as a glassy mass.

Sodium dichromate is the inorganic compound with the formula Na2Cr2O7. However, the salt is usually handled as its dihydrate Na2Cr2O7·2H2O. Virtually all chromium ore is processed via conversion to sodium dichromate and virtually all compounds and materials based on chromium are prepared from this salt. In terms of reactivity and appearance, sodium dichromate and potassium dichromate are very similar. The sodium salt is, however, around twenty times more soluble in water than the potassium salt (49 g/L at 0 °C) and its equivalent weight is also lower, which is often desirable.

Sodium phosphide is the inorganic compound with the formula Na3P. It is a black solid. It is often described as Na+ salt of the P3− anion. Na3P is a source of the highly reactive phosphide anion. It should not be confused with sodium phosphate, Na3PO4.

Calcium arsenate is the inorganic compound with the formula Ca3(AsO4)2. A colourless salt, it was originally used as a pesticide and as a germicide. It is highly soluble in water, in contrast to lead arsenate, which makes it more toxic. Two minerals are hydrates of calcium arsenate: rauenthalite Ca3(AsO4)2·10H2O and phaunouxite Ca3(AsO4)2·11H2O. A related mineral is ferrarisite (Ca5H2(AsO4)4·9H2O.

Sodium arsenate is the inorganic compound with the formula Na3AsO4. Related salts are also called sodium arsenate, including Na2HAsO4 (disodium hydrogen arsenate) and NaH2AsO4 (sodium dihydrogen arsenate). The trisodium salt is a white or colourless solid that is highly toxic. It is usually handled as the dodecahydrate Na3AsO4.12H2O.

Sodium perrhenate (also known as sodium rhenate(VII)) is the inorganic compound with the formula NaReO4. It is a white salt that is soluble in water. It is a common precursor to other rhenium compounds. Its structure resembles that of sodium perchlorate and sodium permanganate.

Ammonium arsenate is the inorganic compound with the formula (NH4)3AsO4. It is prepared by treating a concentrated solution of arsenic acid with ammonia, resulting in precipitation of colorless crystals of the trihydrate. Upon heating, it releases ammonia.
Aluminium arsenate is an inorganic compound with the formula AlAsO4. It is most commonly found as an octahydrate. It is a colourless solid that is produced by the reaction between sodium arsenate and a soluble aluminium salt. Aluminium arsenate occurs naturally as the mineral mansfieldite. Anhydrous form is known as an extremely rare, fumarolic mineral alarsite A synthetic hydrate of aluminium arsenate is produced by hydrothermal method. with the formulation Al2O3·3As2O5·10H2O.
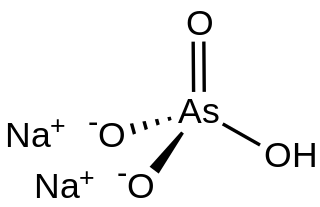
Disodium hydrogen arsenate is the inorganic compound with the formula Na2HAsO4.7H2O. The compound consists of a salt and seven molecules of water of crystallization although for simplicity the formula usually omits the water component. The other sodium arsenates are NaH2AsO4 and Na3AsO4, the latter being called sodium arsenate. Disodium hydrogen arsenate is highly toxic. The salt is the conjugate base of arsenic acid. It is a white, water-soluble solid.
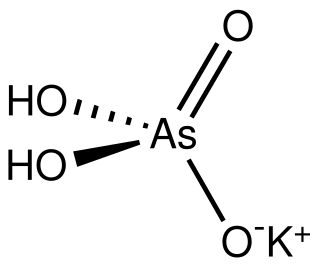
Monopotassium arsenate is the inorganic compound with the formula KH2AsO4. A white solid, this salt is used to prepared other arsenic-containing compounds, mainly pesticides. It is prepared by calcining arsenic oxide and potassium nitrate, followed by extraction with water.
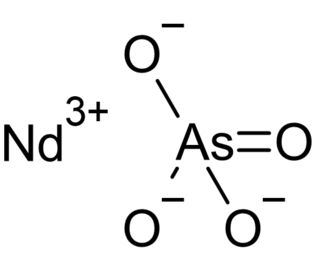
Neodymium arsenate, also known as neodymium(III) arsenate, is the arsenate of neodymium with the chemical formula of NdAsO4. In this compound, neodymium exhibits the +3 oxidation state. It has good thermal stability, and its pKsp,c is 21.86±0.11.

Praseodymium arsenate is the arsenate salt of praseodymium, with the chemical formula of PrAsO4. It has good thermal stability. Its ferroelectric transition temperature is 52°C.

Europium(III) arsenate is an arsenate salt of europium, with the chemical formula of EuAsO4. It has good thermal stability, with its pKsp,c of 22.53±0.03. It is a colorless crystal with a xenotime structure.


















