
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them.
Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms, which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.
Benzonitrile is the chemical compound with the formula C6H5(CN), abbreviated PhCN. This aromatic organic compound is a colorless liquid with a sweet bitter almond odour. It is mainly used as a precursor to the resin benzoguanamine.

Zinc chloride is an inorganic chemical compound with the formula ZnCl2·nH2O, with n ranging from 0 to 4.5, forming hydrates. Zinc chloride, anhydrous and its hydrates, are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four polymorphs of anhydrous zinc chloride.
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes.

Copper(II) chloride, also known as cupric chloride, is an inorganic compound with the chemical formula CuCl2. The monoclinic yellowish-brown anhydrous form slowly absorbs moisture to form the orthorhombic blue-green dihydrate CuCl2·2H2O, with two water molecules of hydration. It is industrially produced for use as a co-catalyst in the Wacker process.

Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures.

Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.

Indium(III) chloride is the chemical compound with the formula InCl3 which forms a tetrahydrate. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium. This is one of three known indium chlorides.
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.
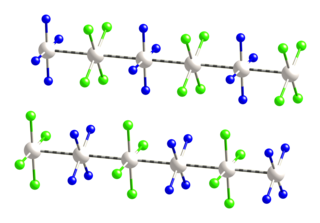
Magnus's green salt is the inorganic compound with the formula [Pt(NH3)4][PtCl4]. This salt is named after Heinrich Gustav Magnus, who, in the early 1830s, first reported the compound. The compound is a linear chain compound, consisting of a chain of platinum atoms. It is dark green, which is unusual for platinum compounds.

Trimethylphosphine is an organophosphorus compound with the formula P(CH3)3, commonly abbreviated as PMe3. This colorless liquid has a strongly unpleasant odor, characteristic of alkylphosphines. The compound is a common ligand in coordination chemistry.

Allylpalladium(II) chloride dimer (APC) is a chemical compound with the formula [(η3-C3H5)PdCl]2. This yellow air-stable compound is an important catalyst used in organic synthesis. It is one of the most widely used transition metal allyl complexes.
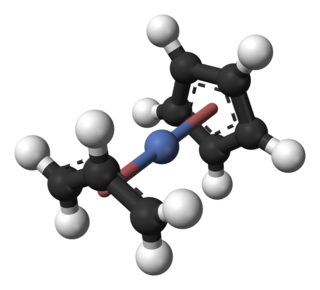
Cyclopentadienyl allyl palladium is an organopalladium compound with formula (C5H5)Pd(C3H5). This reddish solid is volatile with an unpleasant odor. It is soluble in common organic solvents. The molecule consists of a Pd centre sandwiched between a Cp and allyl ligands.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride.

trans-Bis(dinitrogen)bis[1,2-bis(diphenylphosphino)ethane]molybdenum(0) is a coordination complex with the formula Mo(N2)2(dppe)2. It is a relatively air stable yellow-orange solid. It is notable as being the first discovered dinitrogen containing complex of molybdenum.

Bis(benzonitrile)palladium dichloride is the coordination complex with the formula PdCl2(NCC6H5)2. It is the adduct of two benzonitrile (PhCN) ligands with palladium(II) chloride. It is a yellow-brown solid that is soluble in organic solvents. The compound is a reagent and a precatalyst for reactions that require soluble Pd(II). A closely related compound is bis(acetonitrile)palladium dichloride.

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.
Palladium forms a variety of ionic, coordination, and organopalladium compounds, typically with oxidation state Pd0 or Pd2+. Palladium(III) compounds have also been reported. Palladium compounds are frequently used as catalysts in cross-coupling reactions such as the Sonogashira coupling and Suzuki reaction.




















