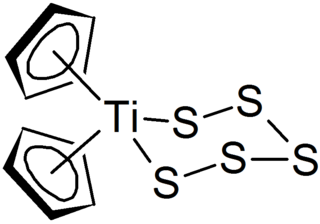Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them.
Sulfide (also sulphide in British English ) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.
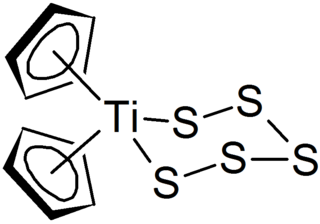
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula S2−
n. These anions are the conjugate bases of polysulfanes H2Sn. Organic polysulfides generally have the formulae R1SnR2, where R is an alkyl or aryl group.

Laurite is an opaque black, metallic ruthenium sulfide mineral with formula: RuS2. It crystallizes in the isometric system. It is in the pyrite structural group. Though it's been found in many localities worldwide, it is extremely rare.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.
Sulfur compounds are chemical compounds formed the element sulfur (S). Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gases.

Copper monosulfide is a chemical compound of copper and sulfur. It was initially thought to occur in nature as the dark indigo blue mineral covellite. However, it was later shown to be rather a cuprous compound, formula Cu+3S(S2). CuS is a moderate conductor of electricity. A black colloidal precipitate of CuS is formed when hydrogen sulfide, H2S, is bubbled through solutions of Cu(II) salts. It is one of a number of binary compounds of copper and sulfur (see copper sulfide for an overview of this subject), and has attracted interest because of its potential uses in catalysis and photovoltaics.
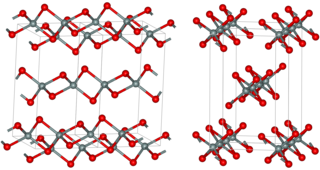
Silicon disulfide is the inorganic compound with the formula SiS2. Like silicon dioxide, this material is polymeric, but it adopts a 1-dimensional structure quite different from the usual forms of SiO2.

Thorium(IV) sulfide (ThS2) is an inorganic chemical compound composed of one thorium atom ionically bonded to two atoms of sulfur. This salt is dark brown and has a melting point of 1905 °C. ThS2 adopts the same orthorhombic lattice structure as PbCl2.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.
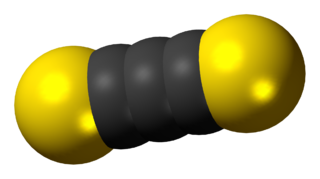
Carbon subsulfide is an organic, sulfur-containing chemical compound with the formula C3S2 and structure S=C=C=C=S. This deep red liquid is immiscible with water but soluble in organic solvents. It readily polymerizes at room temperature to form a hard black solid.
Copper sulfides describe a family of chemical compounds and minerals with the formula CuxSy. Both minerals and synthetic materials comprise these compounds. Some copper sulfides are economically important ores.

Braggite is a sulfide mineral of platinum, palladium and nickel with chemical formula: S. It is a dense, steel grey, opaque mineral which crystallizes in the tetragonal crystal system. It is the central member in the platinum group end-members cooperite and vysotskite.

Titanium disulfide is an inorganic compound with the formula TiS2. A golden yellow solid with high electrical conductivity, it belongs to a group of compounds called transition metal dichalcogenides, which consist of the stoichiometry ME2. TiS2 has been employed as a cathode material in rechargeable batteries.

Platinum disulfide is the inorganic compound with the formula PtS2. It is a black, semiconducting solid, which is insoluble in all solvents. The compound adopts the cadmium iodide structure, being composed of sheets of octahedral Pt and pyramidal sulfide centers. Single crystals are grown by chemical vapor transport using phosphorus as the transport agent. A related compound is platinum(II) sulfide, PtS.

Transition metal thioether complexes comprise coordination complexes of thioether (R2S) ligands. The inventory is extensive.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.
Palladium disulfide is a chemical compound of palladium and sulfur with the chemical formula PdS2.
Palladium sulfide may refer to:
Palladium forms a variety of ionic, coordination, and organopalladium compounds, typically with oxidation state Pd0 or Pd2+. Palladium(III) compounds have also been reported. Palladium compounds are frequently used as catalysts in cross-coupling reactions such as the Sonogashira coupling and Suzuki reaction.