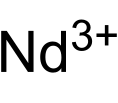Holmium is a chemical element; it has symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like many other lanthanides, holmium is too reactive to be found in native form, as pure holmium slowly forms a yellowish oxide coating when exposed to air. When isolated, holmium is relatively stable in dry air at room temperature. However, it reacts with water and corrodes readily, and also burns in air when heated.
The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.

Praseodymium is a chemical element; it has symbol Pr and the atomic number 59. It is the third member of the lanthanide series and is considered one of the rare-earth metals. It is a soft, silvery, malleable and ductile metal, valued for its magnetic, electrical, chemical, and optical properties. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).

Ytterbium(III) oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It occurs naturally in trace amounts in the mineral gadolinite. It was first isolated from this in 1878 by Jean Charles Galissard de Marignac.

Copper monosulfide is a chemical compound of copper and sulfur. It was initially thought to occur in nature as the dark indigo blue mineral covellite. However, it was later shown to be rather a cuprous compound, formula Cu+3S(S2). CuS is a moderate conductor of electricity. A black colloidal precipitate of CuS is formed when hydrogen sulfide, H2S, is bubbled through solutions of Cu(II) salts. It is one of a number of binary compounds of copper and sulfur (see copper sulfide for an overview of this subject), and has attracted interest because of its potential uses in catalysis and photovoltaics.

Cerium is a chemical element; it has symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the oxidation state of +3 characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure.

Europium(II) sulfide is the inorganic compound with the chemical formula EuS. It is a black, air-stable powder. Europium possesses an oxidation state of +II in europium sulfide, whereas the lanthanides exhibit a typical oxidation state of +III. Its Curie temperature (Tc) is 16.6 K. Below this temperature EuS behaves like a ferromagnetic compound, and above it exhibits simple paramagnetic properties. EuS is stable up to 500 °C in air, when it begins to show signs of oxidation. In an inert environment it decomposes at 1470 °C.

Gallium(III) sulfide, Ga2S3, is a compound of sulfur and gallium, that is a semiconductor that has applications in electronics and photonics.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.

Californium(III) chloride is an inorganic compound with a chemical formula CfCl3. As in californium oxide (Cf2O3) and other californium halides, including californium(III) fluoride (CfF3) and iodide (CfI3), the californium atom has an oxidation state of +3.

Cerium(III) sulfide, also known as cerium sesquisulfide, is an inorganic compound with the formula Ce2S3. It is the sulfide salt of cerium(III) and exists as three polymorphs with different crystal structures.

Neodymium(III) acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.

Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as NdCl3, Nd2(SO4)3 and Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as NdCl2 and NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.
Einsteinium compounds are compounds that contain the element einsteinium (Es). These compounds largely have einsteinium in the +3 oxidation state, or in some cases in the +2 and +4 oxidation states. Although einsteinium is relatively stable, with half-lives ranging from 20 days upwards, these compounds have not been studied in great detail.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.

Holmium(III) sulfide is the sulfide of holmium, with the chemical formula of Ho2S3. Like other rare earth sulfides, it is used as a high-performance inorganic pigment.
Lanthanide compounds are compounds formed by the 15 elements classed as lanthanides. The lanthanides are generally trivalent, although some, such as cerium and europium, are capable of forming compounds in other oxidation states.