
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and more frequently in the past) been applied to all compounds containing covalently bound H atoms. In this broad and potentially archaic sense, water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. In covalent compounds, it implies hydrogen is attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.
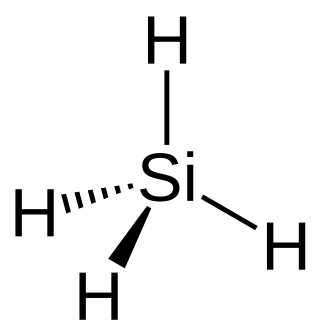
Silane (Silicane) is an inorganic compound with chemical formula SiH4. It is a colorless, pyrophoric gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silanes with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. They are commonly used to apply coatings to surfaces or as an adhesion promoter.

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
Borderline hydrides typically refer to hydrides formed of hydrogen and elements of the periodic table in group 11 and group 12 and indium (In) and thallium (Tl). These compounds have properties intermediate between covalent hydrides and saline hydrides. Hydrides are chemical compounds that contain a metal and hydrogen acting as a negative ion.

Aluminium hydride refers to a collection of inorganic compounds with the formula AlH3. As a gas, alane is a planar molecule. When generated in ether solutions, it exists as an ether adduct. Solutions of alane polymerizes to a solid, which exists in several crystallograhically distinguishable forms.

Plumbane is an inorganic chemical compound with the chemical formula PbH4. It is a colorless gas. It is a metal hydride and group 14 hydride composed of lead and hydrogen. Plumbane is not well characterized or well known, and it is thermodynamically unstable with respect to the loss of a hydrogen atom. Derivatives of plumbane include lead tetrafluoride, PbF4, and tetraethyllead, (CH3CH2)4Pb.
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).

Beryllium hydride is an inorganic compound with the chemical formula n. This alkaline earth hydride is a colourless solid that is insoluble in solvents that do not decompose it. Unlike the ionically bonded hydrides of the heavier Group 2 elements, beryllium hydride is covalently bonded.
Zinc hydride is an inorganic compound with the chemical formula ZnH2. It is a white, odourless solid which slowly decomposes into its elements at room temperature; despite this it is the most stable of the binary first row transition metal hydrides. A variety of coordination compounds containing Zn–H bonds are used as reducing agents, but ZnH2 itself has no common applications.
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.
Cadmium hydride is an inorganic compound with the chemical formula (CdH
2)
n. It is a solid, known only as a thermally unstable, insoluble white powder.
Mercury(I) hydride is an inorganic compound with the chemical formula HgH. It has not yet been obtained in bulk, hence its bulk properties remain unknown. However, molecular mercury(I) hydrides with the formulae HgH and Hg
2H
2 have been isolated in solid gas matrices. The molecular hydrides are very unstable toward thermal decomposition. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.
Titanium(IV) hydride is an inorganic compound with the empirical chemical formula TiH
4. It has not yet been obtained in bulk, hence its bulk properties remain unknown. However, molecular titanium(IV) hydride has been isolated in solid gas matrices. The molecular form is a colourless gas, and very unstable toward thermal decomposition. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.

Chromium(I) hydride, systematically named chromium hydride, is an inorganic compound with the chemical formula (CrH)
n. It occurs naturally in some kinds of stars where it has been detected by its spectrum. However, molecular chromium(I) hydride with the formula CrH has been isolated in solid gas matrices. The molecular hydride is very reactive. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.
Chromium(II) hydride, systematically named chromium dihydride and poly(dihydridochromium) is pale brown solid inorganic compound with the chemical formula (CrH2)n. Although it is thermodynamically unstable toward decomposition at ambient temperatures, it is kinetically metastable.
Iron(II) hydride, systematically named iron dihydride and poly(dihydridoiron) is solid inorganic compound with the chemical formula (FeH
2)
n (also written ([FeH
2])n or FeH
2). ). It is kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it is known as a black, amorphous powder, which was synthesised for the first time in 2014.

Magnesium monohydride is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride.
Barium hydride is a chemical compound with the chemical formula BaH2.
Germyl, trihydridogermanate(1-), trihydrogermanide, trihydridogermyl or according to IUPAC Red Book: germanide is an anion containing germanium bounded with three hydrogens, with formula GeH−3. Germyl is the IUPAC term for the –GeH3 group. For less electropositive elements the bond can be considered covalent rather than ionic as "germanide" indicates. Germanide is the base for germane when it loses a proton.
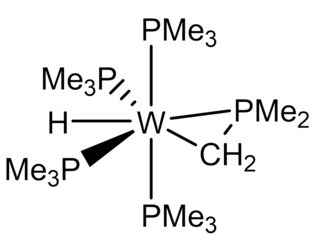
Tetrakis(trimethylphosphine)tungsten(II) trimethylphospinate hydride (W(PMe3)4(η2-CH2PMe2)H) is an air-sensitive organotungsten complex with tungsten in the oxidation state of +2. It is an electron-rich tungsten center is and, thus, prone to oxidation. This bright-yellow complex has been used as a starting retron for some challenging chemistry, such as C-C bond activation, tungsten-chalcogenide multiple bonding, tungsten-tetrel multiple bonding, and desulfurization.









